Studying Hallmarks of Cancer With Recombinant Antibodies
Cancer research is dependent upon reliable antibodies and assays. The output of data from genomic, proteomic, and epigenomic studies comparing tumor and non-tumor cells points to several key traits or “hallmarks”, shared by most tumor types, which drive disease progression. Read more about the hallmarks of cancer and easily find recombinant antibodies specifically targeted to each hallmark.
What Are The Hallmarks Of Cancer?
The hallmarks of cancer are a set of fundamental principles that help break down the intricacies of cancer to better comprehend it. Our knowledge of cancer is rooted in identifying the phenotypic differences between cancer cells and corresponding normal cells of the same lineage. To help us understand these differences, Hanahan and Weinberg published a highly influential review where they organized the complexities of cancer biology into six major hallmarks.1 A decade later, they added two more emerging cancer hallmarks and two enabling traits.2 These are now known as the 10 hallmarks of cancer.
These hallmarks are a set of functional capabilities acquired by mammalian cells as they change from normal disease-free status to neoplastic growth state and acquire specific capabilities that are crucial for their ability to survive and metastasize. Since then, even more emerging hallmarks have been proposed.3
Here we explore 10 hallmarks of cancer and provide a carefully curated list of reliable recombinant antibodies for these targeted studies. Click below to go directly to your cancer hallmark of interest.
Section Overview
- Self-Sufficiency In Proliferation Signals
- Insensitivity To Proliferation-Inhibiting Signals
- Evading Apoptosis
- Enabling Replicative Immortality
- Inducing Angiogenesis
- Tissue Invasion and Metastasis
- Metabolic Reprogramming
- Escape from Immune Control
- Genome Instability and Mutations
- Tumor Promoting Inflammation
Self-Sufficiency In Proliferation Signals
One hallmark of cancer is self-sufficiency in proliferation signals. The normal cell cycle is a tightly regulated process that controls cell proliferation to maintain tissue homeostasis. Even in the presence of growth signals, regulatory proteins keep cell division in check. However, in cancer cells this process is disrupted. For cancer cells to grow, deregulation of the cell cycle and disruption of checkpoints is important. Overexpression of certain receptors and growth promoting ligands and the inactivation of growth restraining tumor suppressor genes allow cancer cells to achieve proliferative advantage.
A common example is the overstimulation of PI 3-kinase/Akt signaling that can perturb the control mechanisms for cell growth and survival and contribute to metastatic competence and resistance to chemotherapy. Abnormal activation of PI 3-kinase is seen in several forms of cancer. About 30% of solid tumors contain mutations in the catalytic unit of their PI 3-kinase, which increases its enzymatic activity and produces excessive Akt signaling. Several oncogenes and tumor suppressor genes that function upstream of Akt are reported to influence cancer progression by affecting Akt activity. Overexpression of Akt1 is reported in various breast cancer cell lines and is important in estrogen-stimulated growth.
Insensitivity To Proliferation-Inhibiting Signals
A second hallmark of cancer is insensitivity to proliferation-inhibiting signals. The evasion of anti-growth signaling is an important feature of cancer cells. To facilitate their growth and proliferation, cancer cells uncouple themselves from several suppressive signals that keep cell proliferation in check. Mutations in cancer suppressive genes are reported in several human cancers. Loss of growth control mechanisms allows neoplastic cells to acquire unlimited replicative ability and evade elimination, growth arrest, and senescence by tumor suppressors.
A common example is TGF-β signaling that is best known for its antiproliferative effects. In many late-stage tumors, TGF- β signaling is redirected away from suppressing cell proliferation and is found instead to activate epithelial-to-mesenchymal transition (EMT) that confers traits associated with high-grade malignancy. It also suppresses immune surveillance, thereby promoting tumor progression.
Retinoblastoma (Rb) proteins that act as gatekeepers of cell cycle progression are reported to be defective in several cancers. Defects in PTEN, another major tumor suppressor that blocks the PI-3 kinase/Akt signaling pathway by removing the phosphate in the 3-position on PIP3, are also reported in several human cancers.
Evading Apoptosis
A third hallmark of cancer is evading apoptosis. Tumor cells expand their population by two mechanisms- by increasing the rate of cell proliferation and by blocking the rate of cell death via apoptosis. The apoptotic machinery consists of sensors and effectors that jointly monitor the extracellular and intracellular factors that determine if the cell should survive or die. The ultimate effectors of apoptosis are caspases that are activated in a very controlled manner. Two “gatekeeper” caspases, −8 and −9, are activated by death receptors such as FAS or by the cytochrome C released from mitochondria, respectively. These two proximal caspases trigger the activation of several other effector caspases that execute the cell death program, through selective destruction of subcellular structures and organelles, and of the genome (Figure 1).
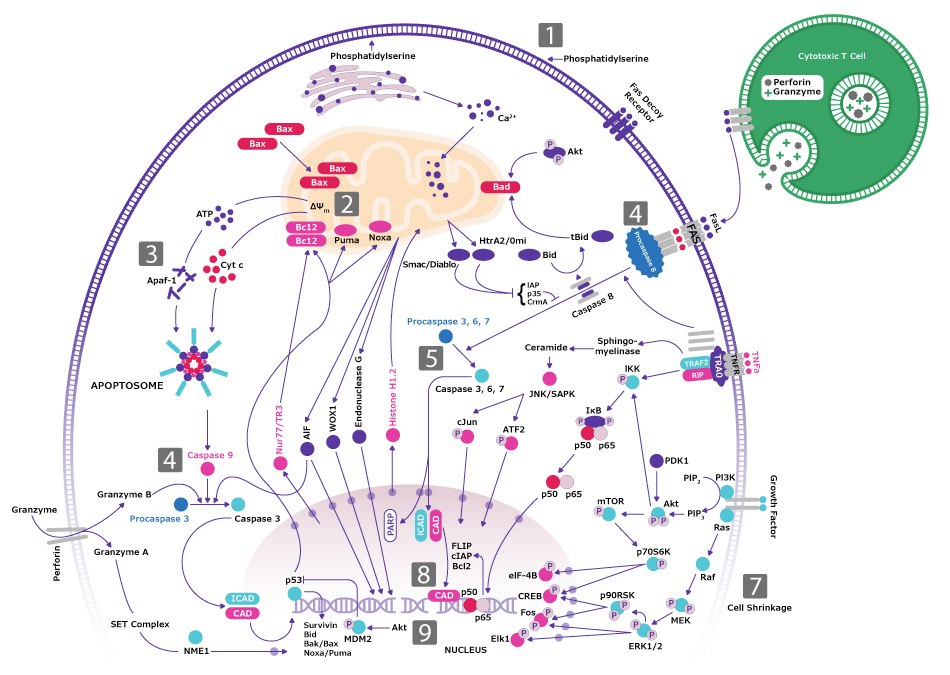
Figure 1.Apoptosis signaling pathway highlighting the gatekeepers, caspase-8 and caspase-9, executing the cell death program.
Cancer cells evade apoptosis via multiple mechanisms. Most commonly, they exhibit mutations or deletions of tumor suppressor genes. They may also have diminished function of proapoptotic proteins, such as Bax or Puma, and show overexpression of anti-apoptotic Bcl-2 family proteins. Cancer cells may also alter the mechanisms that detect DNA damage or irregularities, preventing proper signaling and apoptosis activation.
Enabling Replicative Immortality
A fourth hallmark of cancer is enabling replicative immortality. When telomeres reach a critical minimum length, cells fail to divide, and cellular senescence and apoptosis sets in. Stem cells, germ cells, cancer cells, and certain somatic cells overcome telomere shortening by expressing telomerase, which allows them to maintain their telomere length. Telomerase preserves telomere length in stem cells, germ cells, and cancer cells by adding hexameric (TTAGGG) repeats to the ends of chromosomes, thereby circumventing the cumulative damage that occurs with each mitotic cell division (Figure 2).
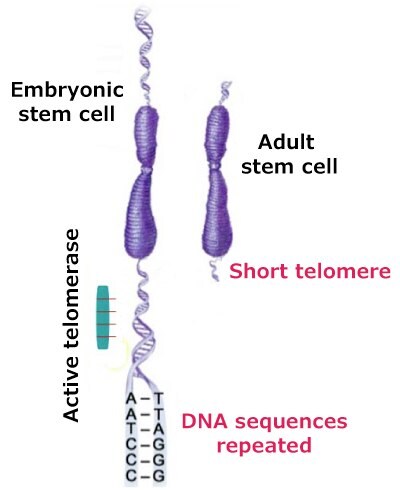
Figure 2.Active telomerase activity adding repeated DNA sequences.
Dysregulation of telomerase expression has been reported in several cancers. About 90% of cancer cells contain short telomeres but exhibit high telomerase activity. For example, 75% of oral carcinomas, 80% of lung cancers, 93% of breast cancers, and 95% of colorectal cancers, have detectable telomerase activity. As cancer cells divide more often, they possess, on an average, shorter telomeres than normal cells. Hence, without an active telomerase to maintain telomere length, cancer cells could reach critically short telomeres at a faster pace than normal cells. The presence of telomerase activity is correlated with poor clinical outcomes.
Inducing Angiogenesis
A fifth hallmark of cancer is inducing angiogenesis. Angiogenesis, the new capillary formation from established vasculature by migration and proliferation of endothelial cells, is a highly regulated process required for a number of physiological and pathological events. Tumor cells often face hypoxia, low pH, and low glucose levels. To overcome these harsh conditions, they secrete angiogenic growth factors that promote neovascularization for tumor growth, invasion, and metastasis. Under normal physiological conditions, angiogenesis is controlled by a delicate balance between the positive and negative regulators.
Most tumors persist for years without any angiogenic activity, incapable of growing beyond 2 to 3 mm in size. These dormant tumors secrete inhibitory factors that prevent them from switching to the angiogenic phenotype. In their dormant stage, the rate of tumor cell proliferation is balanced by apoptosis of tumor cells. For angiogenesis to occur, proangiogenic molecules must overcome the effect of those inhibitory factors. The acquisition of angiogenic phenotype results in a diminution in the apoptotic rate and a shift in balance in favor of cell proliferation. VEGF and basic FGF are the two most potent angiogenic factors involved in endothelial cell survival, proliferation, and migration (Figure 3).
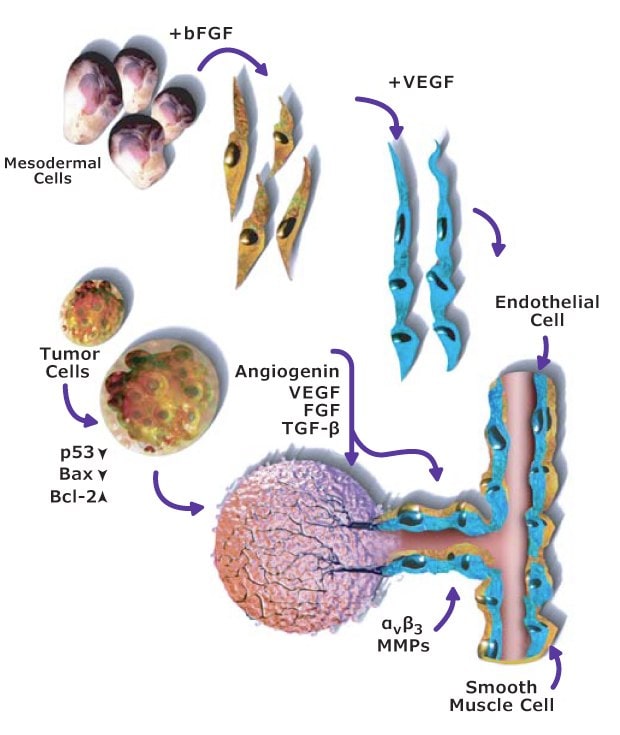
Figure 3.Angiogenesis signaling pathway including the role of VEGF and FGF angiogenic factors.
Most antitumor agents used in cancer therapy are cytotoxic in nature, designed mainly to prevent tumor growth. However, angiogenic inhibitors, due to their selective effect on vasculature, have an overall reduced toxicity, and can overcome drug resistance commonly seen in solid tumors.
Tissue Invasion and Metastasis
A sixth hallmark of cancer is tissue invasion and metastasis. Metastasis is the cumulative result of multiple changes in tumor cells and their microenvironment that enable cells to migrate to locations distant from the primary tumor and colonize the host tissue. The metastatic cascade can be divided into three main processes: invasion, intravasation, and extravasation. The loss of cell-cell adhesion allows malignant tumor cells to dissociate from the primary tumor mass and changes in cell-matrix interactions enable these cells to invade the surrounding stroma. Invasion of the stroma and vessel walls requires tumor cells to secrete matrix-degrading enzymes.
After tumor cells survive circulation, they adhere to endothelial cells at the metastatic site, extravasate, and colonize the new host tissue. The ability to colonize distant sites is mediated both by tumor cell adhesion as well as pre-metastatic changes in the distant microenvironment that prepare for the arrival of metastatic cells. The mechanisms by which this “metastatic niche” is determined are topics of great interest to tumor biologists.
Metabolic Reprogramming
A seventh hallmark of cancer is metabolic reprogramming. Rapidly proliferating cancer cells have higher nutritional demands, and they undergo extraordinary adaptations to meet their needs. Cancer cells alter their ability to metabolize carbohydrates, lipids, and proteins to meet energy demands in changing environmental conditions and changes in accessible substrates. Cancer cells generate a substantial proportion of their ATP via aerobic glycolysis, as opposed to ATP production via oxidative phosphorylation.
The Warburg effect is a well-studied adaptation in which tumor cells express a form of pyruvate kinase that enables them to maintain a glycolytic rate that is ~200 times higher than that of normal cells. It provides energy even when mitochondria are damaged. In addition to increased rates of glycolysis, tumor cells also increase glucose flux through the pentose phosphate pathway (PPP). This pathway generates ribose-5-phosphate that is required for purines and pyrimidines, and NADPH that is essential for lipid and nucleotide synthesis. Depending on their metabolic demands, cancer cells can shunt glucose either via glycolysis, via PPP, or both.
In another metabolic adaptation, cancer cells express mutant isocitrate dehydrogenase (IDH), which convert α-ketoglutarate into 2-hydroxyglutarate. The latter metabolite activates angiogenesis via the HIF-1 pathway and also affects gene expression by repressing histone demethylases. In cancer cells glutamine serves as an important nitrogen source for starved cancer cells, enabling rapid protein and nucleic acid synthesis. This demand is achieved by increasing the number of glutamine transporters.
Escape from Immune Control
An eighth hallmark of cancer is escape from immune control. Tumor growth and metastasis depend on a complex interplay between the host immune system and counter-regulatory immune escape pathways. Our immune system keeps a strong surveillance that recognizes and eliminates malignant cells. However, tumor cells gradually develop mechanisms to escape this immune surveillance, which leads to tumor growth, metastasis, and resistance to immunotherapy.
Tumor cells undergo many genetic and epigenetic changes that result in the formation of neoantigens, that can trigger T cells and generate a population of cytotoxic T lymphocytes (CTLs) that can recognize and kill cancer cells. However, cancer cells overexpress certain checkpoint molecules that inhibit T cell activation and upregulate negative signals to facilitate cancer progression and metastasis. Some tumor cells may also activate immunosuppressive leukocytes to create a tumor microenvironment that poorly responds to antitumor immune molecules.
A common example is the programmed cell death protein 1 (PD-1) that is expressed on surface of all activated T cells. In the tumor microenvironment PD-1 and programmed death ligand 1 and 2 (PD-L1, PD-L2) play a vital role in tumor progression. PD-1 is expressed on several cells of the immune system and on tumor-infiltrating lymphocytes (Figure 4). However, PD-L1 and PD-L2 are overexpressed on tumor cells and their interaction with PD-1 on T cells can disrupt normal T cell activation and allow tumor cells to escape immune surveillance. Several cancer cells are reported to overexpress PD-L1 and PD-L2 to protect themselves and circumvent a delicate balance to avoid any “immune killing” by T cells.
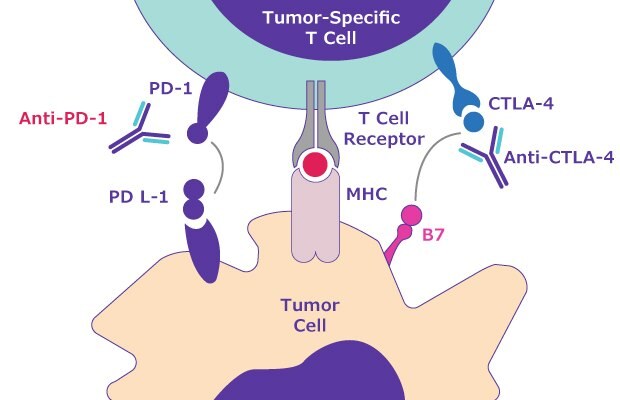
Figure 4.Immune escape signaling pathway including the role of PD-1 and PD-L1/PD-L2.
Genome Instability and Mutations
A ninth hallmark of cancer is genome instability and mutations. Genomic instability is observed in several cancers, and it involves changes in DNA sequence caused by mutation or gene amplification. Genomic instability also results from mutations in DNA repair genes. In normal cells, mutations are efficiently prevented by tumor suppressing systems that repair damaged DNA, inactivate DNA-damaging agents, or promote genomic integrity. However, with defects in if any of the tumor suppressing caretaker genes (such as p53 and ATM) and mitotic checkpoint genes (such as p16 and p14) cells experience increased mutations, chromosomal damage, recombination, and possibly aneuploidy. With genome sequence data now available from thousands of tumors, hypermutation signatures are becoming known and have revealed mechanisms of carcinogenesis, which includes effect of certain food additives, pollutants, and pathogen infection.
Tumor Promoting Inflammation
A tenth hallmark of cancer is tumor promoting inflammation. Inflammation, a normal physiological response to injury or infection, results in the activation of several pro-inflammatory genes and the release of reactive oxygen and nitrogen species. Although, production and release of free radicals at the site of inflammation is helpful in eliminating invading pathogens, it can also damage healthy epithelial and stromal cells, which may lead to malignancy. Chronic inflammation triggered by extrinsic and intrinsic factors can promote all stages of tumorigenesis, including limitless replication, apoptosis evasion, self-sufficiency in growth signaling, and sustained angiogenesis.
The inflammatory link to carcinogenesis is supported by the fact about 20% of all causes of cancer are linked to chronic inflammation and dysregulated activation of the NF-κB signaling pathway (Figure 5). Several human lymphoid cancer cells are reported to have either mutations or amplifications of genes encoding NF-κB. Constitutively active NF-κB is reported in many types of human tumors and it not only protects cancer cells from apoptotic cell death, but also enhances their growth potential.
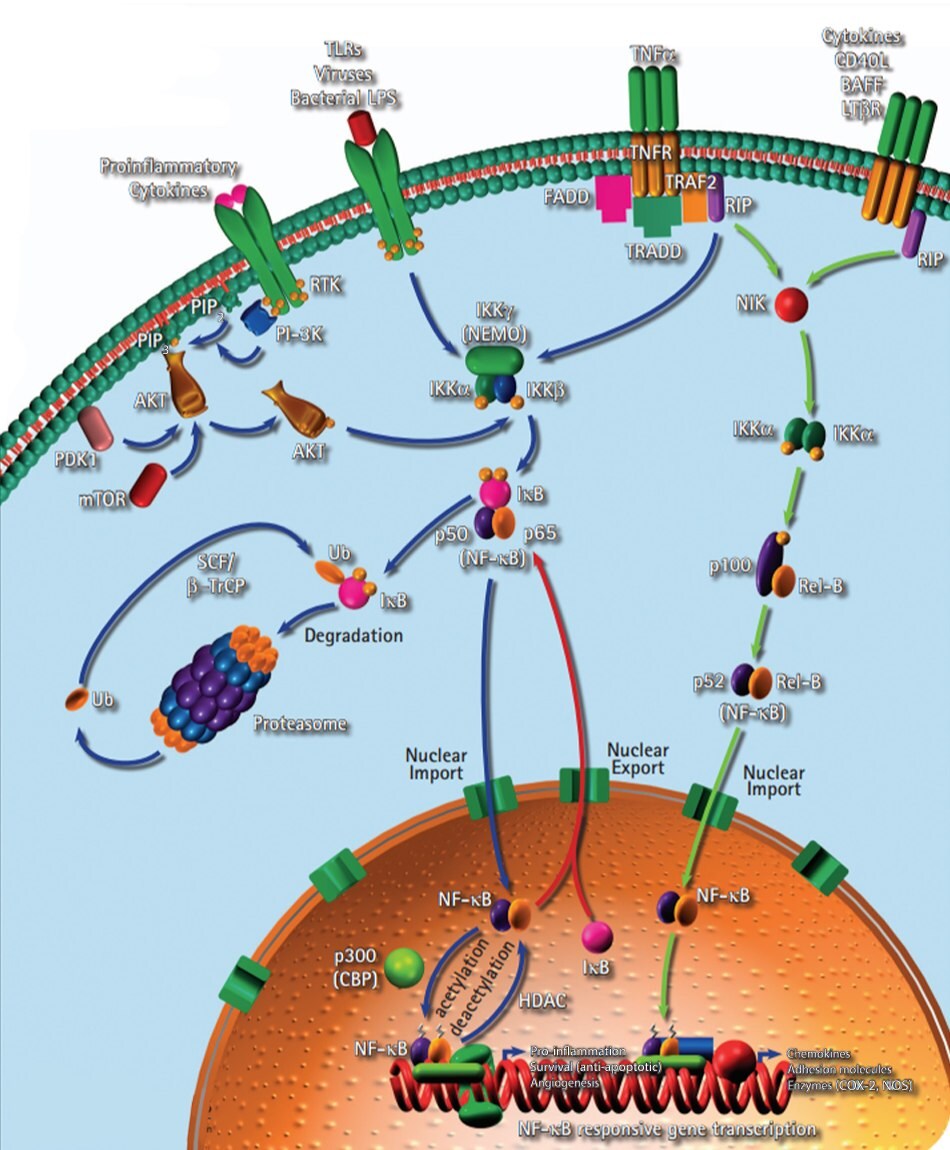
Figure 5.Inflammation signaling pathway including the role of NF-κB.
For Research Use Only. Not For Use In Diagnostic Procedures.
References
Pour continuer à lire, veuillez vous connecter à votre compte ou en créer un.
Vous n'avez pas de compte ?