Development and Optimization of a Lentivirus Manufacturing Platform
Lentivirus-based vectors are widely used to modify T cells for use in cell-based immunotherapies such as chimeric antigen receptor T cell (CAR-T) therapies. In addition to gene-modified cell therapies, viral vectors are essential for developing gene therapies for a wide range of diseases, both rare indications and those affecting large patient populations. The success of these innovative therapies has increased demand for lentiviral vectors and highlighted the need for improved lentivirus manufacturing processes.
Clinical and commercial manufacturing of lentiviral vectors typically relies on transient transfection of four plasmids into adherent HEK293T cells. However, adherent cell cultures present scalability challenges in upstream production of lentivirus viral gene therapy manufacturing processes. To address these challenges, we developed a lentivirus manufacturing production platform based on a suspension adapted HEK293T cell line. This scalable platform delivers high productivity, reduces process development time, enables easy scale up, and accelerates time to market.
This article summarizes the studies performed during to development and upstream process optimization for lentivirus production.
- Development of a Suspension Adapted HEK293T Cell Line
- Development of a Chemically Defined Medium for Lentiviral Production
- Upstream Process Optimization for Lentivirus Production
- Representative Scale-up from Shake flasks to 50 L Bioreactors
- Materials and Methods Used to Optimize the Lentivirus Manufacturing Platform
Figure 1. Challenges faced by lentivirus manufacturers with adherent platform for viral vector production and suspension-based, scalable manufacturing system as a solution to these challenges.
Development of a suspension adapted HEK293T cell line for lentiviral production
The traditional HEK293T cell line is derived from a human embryonic kidney (HEK) cell line containing the SV40 Large T antigen gene. This cell line is typically grown in adherent cultures containing fetal calf serum and has limitations in lentivirus productivity.
An optimized HEK293T cell line was created by cloning the HEK293T cell line and screening individual clones for improved cell growth and lentiviral production. Figure 2 shows the superior virus productivity of clone 3F11 as compared to the parental cell line or other clones. Following selection, the new cell line was passaged and adapted to suspension growth in chemically defined medium.
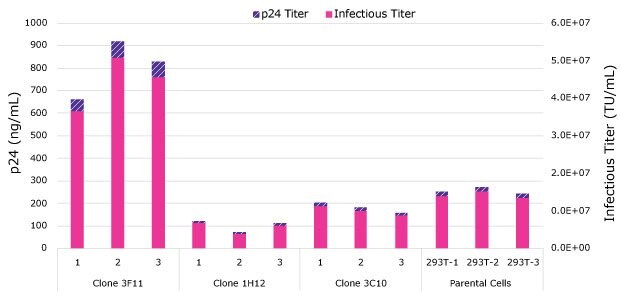
Figure 2.Lentivirus harvest titers at 48 hours post-transfection. Clone 3F11 demonstrated superior lentivirus titers compared to the other clones and parental HEK293T cells.
This clonal, suspension adapted, HEK293T derivative cell line supports high levels of lentivirus production and is more amenable to clinical and commercial lentivirus manufacturing than the adherent cell line. Importantly, this HEK293T VirusExpress® cell line has been banked under cGMP conditions (21 CFR 210, 211, 600, 610) and comprehensively tested for adventitious agents and cell identity.
Development of a chemically defined medium for lentiviral production
To minimize risk, biopharmaceutical manufacturers have moved towards serum-free, animal component-free, and fully chemically defined media for upstream production of both viral vectors and other cell-based therapies. Additional benefits of this shift include more consistent upstream cell culture performance, improved viral productivity (titers), simplified sourcing and often reduced costs.
To realize these benefits for lentivirus manufacturing, a chemically defined medium was developed for use with the clonally derived HEK293T VirusExpress® Cells. Target criteria for the design of this chemically defined, animal origin free medium included:
- Faster cell growth and high cell densities (> 8E06 cells/mL) with minimal clumping of cells in the bioreactor
- High transfection efficiency and compatible with linear PEI, the standard transfection reagent
- High virus productivity
- No medium exchange prior to transfection; a single formulation suitable for cell growth, transfection, and viral production
- Availability at large scale to support clinical and commercial manufacturing
- Only qualified raw materials with supporting regulatory documentation
The EX-CELL® CD HEK293 Viral Vector Medium met these criteria and supported growth of HEK293T VirusExpress® Cells. The medium is available in both liquid and dry powder formats and enables consistent batch-to-batch performance and high lentivirus infectious titers.
Upstream Process Optimization for Lentivirus Production
To optimize lentiviral vector production, a Design of Experiment (DoE) tool was used to evaluate multiple factors that affect transient transfection.
The first study was performed in shake flasks and assessed cell density, plasmid DNA and PEI ratio, complex formation volume, complex formation time, and total plasmid DNA/mL on cell health and infectivity titers (DoE #1). The effect of all parameters on infectious titer was modeled using JMP® software. All main factors had a significant effect on infectious titers with a 16-fold difference between the highest (6.3E+08 TU/mL) and lowest titer (3.8E+07 TU/mL). The optimum conditions were confirmed in additional shake flask studies before scale-up to Mobius® 3 L bioreactors. 48 hours post-transfection, material was harvested and titered. Figure 3 shows lentivirus titers generated from HEK293T VirusExpress® Cells using EX-CELL® CD HEK293 Viral Vector Medium in 3 L Mobius® bioreactors were representative of the small-scale studies in shake flasks.

Figure 3.Lentivirus titers obtained in 3 L bioreactors (BRX) setup with five factors and their optimal levels identified in DoE #1. Each bar represents an average of 2-3 dilutions of the lentivirus harvest sample plated in triplicates and error bars are stdev.
The second DoE was designed to optimize the ratio of the four third- generation plasmids used for lentivirus production. Statistical modelling was used to assess all effects on infectious titers from total of thirty runs over two rounds of experiments. The optimal plasmid molar ratio for each plasmid was identified with the use of the prediction profiler tool (Figure 4).
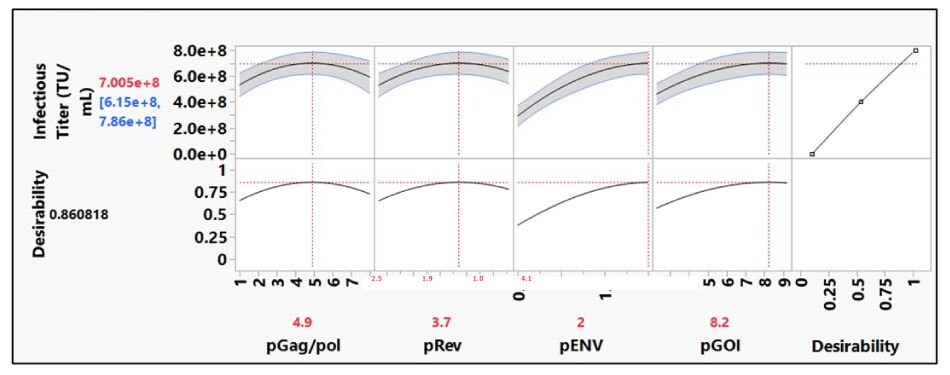
Figure 4.In the prediction profiler tool, the titer response reached a plateau with both pGag/pol and pRev plasmids. For maximum harvest titer desirability, the tool predicted the optimal plasmid molar ratios.
The optimal molar ratio for each plasmid for transfection was confirmed in shake flasks (data not shown) before scale-up to Mobius® 3 L bioreactors.
One of the objectives of the upstream platform process optimization for lentiviral production was to ensure optimal cell health in addition to maximizing infectious titers. Figure 5A shows viability of >90% at 48 hours post-transfection (Day 3) with infectious titers of 5.2E+08 TU/mL to 5.9E+08 TU/mL in two Mobius® 3 L bioreactors (Figure 5B).

Figure 5A.At harvest time point of 48 hours post-transfection (Day 6), VCD of 6E+06 vc/mL to ~8E+06 vc/mL was achieved in Mobius® 3 L Bioreactors (BRX) (solid lines) while maintaining harvest viability of > 90% (dotted lines). Transfection was done on Day 4.

Figure 5B.Scale-up of optimal plasmid molar ratios in Mobius® 3 L Bioreactors. Each bar represents an average of two to three dilutions of the sample plated in triplicates (n=6 or 9) and error bars are stdev.
Representative Scale up from Shake flasks to 50 L Bioreactors
The robust performance of the lentivirus production process in shake flasks and 3 L bioreactors, offered promise for scale-up to 50 L scale. Due to the turndown ratio of 5:1 with Mobius® 50 L bioreactor, cell expansion (N-1 stage) and lentivirus production (N) stages took place in the 50 L single-use bioreactor. Once the desired viable cell density was achieved at the 10 L working volume, fresh EX-CELL® CD HEK293 Viral Vector Medium was added to increase the volume to 40 L. Following plasmid transfection at the identified optimal conditions and 48 hours incubation, lentivirus was harvested. For each of two separate production runs, infectious titer was 3.8 +08 TU/mL and 4.8E+08 TU/mL (Figure 6).

Figure 6.Scale-up of lentivirus production in Mobius®® 50 L Bioreactors (purple bars) and shake flasks (pink bars) as controls. Each bar represents an average of two to three dilutions of the sample plated in triplicates (n=6 or 9) and error bars are standard deviation.
While lentivirus titers in the 50 L bioreactors are slightly lower than the titers in shake flasks, these lentivirus titers generated using a suspension adapted HEK293T cell line are higher than those of adherent cells and represent a significant advantage to biomanufacturers looking to develop and scale-up upstream processes for manufacturing of lentiviral vectors.
Conclusion
The VirusExpress® Lentiviral Platform was developed to address the challenges associated with existing cell culture product systems. This new lentiviral platform represents a significant advance and is based on proven, trusted methodologies:
- A novel HEK293T cell line that is well characterized and GMP banked
- A chemically defined medium compatible with PEI-based transfection and other transfection reagents
- A single medium for growth of cells, transfection, and production steps
- An upstream process optimized for suspension-based lentivirus production at various scales tested with industry leading titers of 4.8E+08 TU/mL to 6.7E+08 TU/mL equating to an increase of 3.3x to 8.0x compared to titers typically reported.
Materials and Methods Used to Optimize the Lentivirus Manufacturing Platform
HEK293T VirusExpress® Cells, adventitious agent tested, and GMP banked (VP001-1VL, SAFC) were used for lentiviral vector production.
EX-CELL® CD HEK293 Viral Vector Medium (14385C, 24385C, SAFC) supplemented with 6 mM L-Glutamine (G7513, SAFC) for seed train maintenance and expansion, transfection, and lentivirus production.
DoE experiments, a baseline lentivirus production process in 125 mL shake flasks was used. Flasks were incubated with a 30 mL working volume at 37 °C, 80% RH, 8% CO2 and 135 rpm (25 mm orbital throw).
Routine seed train maintenance: Cells were passaged at 0.5E+06 vc/mL and cultured for 3 days or at 0.25E+06 vc/mL for 4 days.
Lentivirus production: Cells were seeded at 0.5E+06 vc/mL, 0.7E+06 vc/mL or 0.9E+06 vc/mL for 3 days at N-1 stage for DoE #1. On day three, cells were diluted 1:4 with fresh EX-CELL® CD HEK293 Viral Vector Medium. For DoE #2, 3 L and 50 L bioreactor runs, HEK293T VirusExpress® 293T Cells were seeded at a density recommended by JMP® data modeling from DoE #1 followed by the 1:4 dilution of cells. For both DoE experiments, the 1:4 diluted cells were incubated for 24 hours prior to transfection. Third generation lentivirus plasmid system was used for viral vector production with a lentivirus transfer vector that contained eGFP transgene along with PEIpro® (Polyplus-transfection) transfection reagent. Cell counts and lentivirus harvest samples were collected on days one and 2 post-transfection.
Bioreactor confirmation and scale-up runs in Mobius® 3 L & 50 L Single-Use Bioreactors: Optimal process parameters for pH, agitation, and dissolved oxygen and gassing configurations were set to values determined during process development: dissolved oxygen set to 50%, pH 7.1±0.1 and temperature 37 ˚C.
Lentivirus titer determinations: HT1080 cells expressing red fluorescent protein (RFP) in nuclei were plated in 96 well plates for 24 hours followed by incubation with lentivirus samples pre-diluted with 8 µg/mL of polybrene in growth medium. Two to three dilutions were assayed and for each dilution, triplicate lentivirus samples were added. The plates were incubated at 37 °C and 5% CO2 in a static incubator for 72 hours followed by fluorescence imaging (red and green) using Cytation 5 imager. BioTek Gen5 software was used for image analysis, data processing and reporting steps. Live, green fluorescent protein (GFP) expressing cells were reported as a percentage and this value was used to calculate lentivirus infectious titer based on cell density at time of transduction.
Pour continuer à lire, veuillez vous connecter à votre compte ou en créer un.
Vous n'avez pas de compte ?