What is Hyaluronan?
What is Hyaluronic Acid?
Hyaluronic acid (HA) is the simplest glycosaminoglycan (a class of negatively charged polysaccharides) and a major constituent of the extracellular matrix (ECM).1 Hyaluronan is a scaffold secreted by cells that surrounds them in vivo,2 HA is a linear, non-sulfated polysaccharide that provides compression strength, lubrication and hydration within the ECM,2 It also regulates cell adhesion and motility3,4 and mediates cell proliferation and differentiation5 making it not only a structural component of tissues, but also an active, signaling molecule.
Discover more about 3D cell culture applications of semi-synthetic hyaluronic acid-based 3D hydrogels with the HyStem™ platform.
Nomenclature
- Hyaluronan (refers to all physiological forms of HA, the most common of which is the sodium salt)
- Hyaluronic acid
- Abbreviated most commonly as HA, sometimes as HY
Chemistry and Physical Properties of Hyaluronic Acid
HA is a linear, unbranched, alternating polymer composed of two monosaccharides: ß(1,4)-N-acetyl-D-glucosamine and ß(1,3)-D-glucuronic acid (see Figure).6 It is non-sulfated, unlike other glycosaminoglycans (GAGs) prominent in the ECM, such as heparin and chondroitin sulfate. The chain length of HA varies from ~0.200 to 10 MDa, with the most common sizes ranging from 2-5 MDa.6,7,8
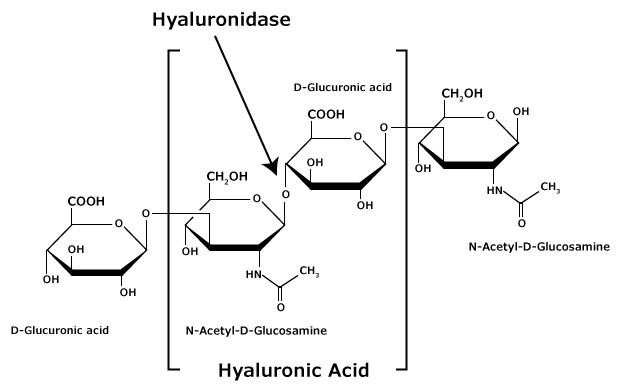
Figure 1.Hyaluronic acid is composed of alternating residues of β-D-(1‑3) glucuronic acid and β-D-(1‑4)-N-acetylglucosamine.
HA dissolves in water to form a viscoelastic solution, with increasing concentration raising the solution viscosity (10 mg/ml has a viscosity 5000x that of water),6 However, under shear stress, the viscosity drops rapidly while maintaining elasticity. This characteristic makes HA ideal as a biological lubricant.9 The actual structure of HA in the ECM varies, but it can be generally characterized as a random coil with an extended conformation and considerable intrinsic stiffness. Hydrogen bonding between adjacent saccharides appears to be the most significant force in setting HA’s physiological properties,9 However, the physical properties of HA in vitro are also significantly influenced by ionic strength.9
Find more technical resources on our Glycobiology hub page.
Biological Roles
HA interacts with a variety of ECM molecules, making it an essential structural component,7 It is continuously secreted from fibroblasts, keratinocytes, chondrocytes and other specialized cells throughout the body and degraded by enzymes (hyaluronidases).10 The amount of HA present in various tissues and fluids in humans and its half life and molecular weight range are listed in the table below.
Structural2,6
HA, along with the other GAGs present in the ECM, provides compressive strength to tissues. Its negative charge, hydrophilicity and long polymer length result in large amounts of water being bound with the matrix. Additionally, HA’s unique property of lowering its viscosity during shear stress means it works as a lubricant. Under static conditions, it provides resiliency. Finally, in addition to providing a hydrated space around cells, it regulates the traffic of growth factors and other signals due to its pore size and charge density.
Signaling
HA has six characterized cell surface receptors:12,13
- CD44
- RHAMM (receptor for hyaluronan mediated motility), CD168
- LYVE-1 (lymphatic vessel endothelial HA receptor-1)
- HARE (hyaluronan receptor for endocytosis)
- layilin
- Toll-4
- CD44 and RHAMM are the best characterized to date. Both are thought to be involved in tumor migration, invasion, adhesion and proliferation.11-13
CD44
CD44 is a Type I single pass, transmembrane protein.11,13 The smallest HA unit that could competitively bind longer HA polymers in CD44 is a decasaccharide.7 CD44 is abundantly expressed by many cell types. It has 17 isoforms. It has even more heterogeneity due to various post-translational modifications (glycosylation, phosphorylation, and protein palmitoylation). CD44 has been shown to have a vast array of cellular functions, including functioning as an:13
- HA Receptor
- Integral membrane proteoglycan
- Docking protein for matrix metalloproteinases
- Nuclear transcription factor
- Signal transducer for actin cytoskeleton
- Manager of HA-rich ECM
- Mediator of adhesion and lymphocyte rolling
- Coordinator of matrix signals for cell survival/death
- RHAMM or CD16812
RHAMM (receptor for hyaluronan mediated motility) is not a transmembrane protein. Instead, it associates with the cell surface, but is present in several cellular compartments. RHAMM also binds to sulfated GAGs, like heparin. To date, this HA binding protein has been shown to be involved in:
- PDGF and HA-mediated activation of SRC, FAK, PKC and ERK signaling cascades
- Motility in response to wounding
- Progression through G2M of the cell cycle
- Tubule formation during angiogenesis
Development/Embryogenesis
“Although it is essentially ignored in most descriptions of tissue development, one of the most important cellular behaviors that permits the formation of the first specialized tissue forms (i.e., planar ectoderm and endoderm) is the production of the extracellular matrix attachment scaffolds that hold together cells within all solid tissues”.13
The ECM plays a vital role not just in homeostasis of tissues, but also in their development. As a new epithelium is formed, a basement membrane is made at the same time. This includes when an epithelium is made during the generation of the ectoderm and endoderm within the embryo. During embryogenesis, laminin is the first ECM protein to be excreted within the basement membrane. It is observed in a punctuate pattern in the intercellular spaces between cells in the 8 cell stage embryo. Later in development, fibronectin, heparan sulfate and collagen IV accumulate in the same area. The deposition and self-assembly of collagen IV leads to the organization of the basement membrane and hence to the organization of the attached cells, leading to the polarization of the epithelial monolayer.13
Embryonic ECM possesses a high quantity of glycosaminoglycans (GAGs), of which HA is predominant.14 Human embryonic stem cells (H1, H9 and H13 lines) have been shown to express both CD44 and CD168 (RHAMM), which are HA receptors. Human embryonic stem cells (hESCs) have also been cultivated by encapsulating them in HA hydrogels. These cells were grown for 15 days without detectable differentiation. After recovery from the hydrogels, the hESCs could be differentiated using endothelial growth media supplemented with VEGF.14
HA is present in large amounts in the ECM of embryonic livers, fetal livers and the stem cell niche within the liver (the Canals of Hering). Hepatic stem and progenitors cells (hepatoblasts) have been successfully cultured for over 4 weeks without differentiation when encapsulated in HA hydrogels and grown with a fully defined media (Kubota’s medium). Additionally, these hepatic progenitor cells express CD44 at high levels.15
Cancer
The role that HA plays in cancer is still being elucidated. To date, fourteen types of carcinoma have been shown to have elevated levels of hyaluronan in the tumor cells or the surrounding stroma,16,19 However, whether this is a causal relationship or not is unclear. For ovarian cancer, the correlation between the accumulation of HA and cancer is so strong that it is being considered as a prognostic marker.19 Low MW HA can stimulate migration of tumor cells (high MW HA cannot),19 Recently, inhibition of endogenous HA synthesis (by transfection of antisense-HAS, hyaluronan synthase) has been shown to dramatically reduce PC3M-LN4 (human prostate carcinoma) tumor growth in vivo when injected subcutaneously in immunocompromised mice. These same tumors showed 70-80% lower blood vessel densities. Tumor growth was restored by injecting the tumors with exogenous, high molecular weight HA16. Additionally, HA expression has also been shown to affect several signaling pathways (Erb2, Ras, MAPK, and PI3 kinase/Akt) that promote tumor growth and survival.16
Angiogenesis
HA oligosaccharides promote angiogenesis, while high molecular weight HA retards it.16,17,19,20 Besides HA fragments and polymers affecting angiogenesis, hyaluronidase levels and HA degradation correlate to tumor growth and angiogenesis. Therefore, it is inferred that degradation of tumor HA generates HA oligosaccharides, which in turn stimulates angiogenesis and tumor growth. There is some contradictory information to this theory, indicating that more research is required to fully understand the complicated relationship between HA and vascularization.16
Degradation of Hyaluronan
HA is removed from an organism by two routes, both of which require hyaluronidases10,20:
1. Internalization and degradation by cells and destruction in the lyosome10:
The hyaluronidase, Hyal-1, is responsible for the catabolism of intracellular HA and functions primarily in the lyosome.10,20 This is the enzyme that creates angiogenic HA fragments since it cleaves HA chains of all sizes down to tetrasaccharides,20-15 Degradation of the tetrasaccharides created by Hyal-1 is completed by β-glucuronidases and β-N-acetyl-glucosaminidases in the lyosome. The final degradation products are GlcNAc (which can be recycled) and GlcA (which is catabolized in the pentose pathway).6
2. Release from the ECM and drainage into the vasculature, followed by removal by the lymph nodes, liver and kidney10:
The hyaluronidase, Hyal-2, is responsible for breaking down extracellular HA.10,20 After being cleaved, HA is transported through the lymphatic system. Once in the blood stream, the liver removes about 80% and the kidney another 10%.10
Enzymatic7
There are three classifications of hyaluronidases that digest HA:
- mammalian (endo-β-N-acetyl-D-hexosaminidases that make tetra- and hexasaccharides)
- leeches/parasite (endo-β-glucuronidases)
- bacterial (act through β-elimination to make di-, tetra- or hexasaccharides; note: this enzyme introduces a double bond in the uronic acid at the non-reducing end which is detectable at 232 nm).
Hyaluronidases function best at acidic pH, which is expected given their role in lyosomal degradation. Enzymatic digestions are typically done in sodium acetate buffer (pH 4.8-6.0) at 37 °C. By varying the time of digestion, the resulting pool of HA oligosaccharides changes – the longer the digestion, the shorter the chain lengths.
There are 5 homologous mammalian types of hyaluronidases encoded in the human genome: Hyal-1 to 4 and PH-20 (sperm adhesion molecule 1, or SPAM-1).20
- Hyal-1 is expressed in most tissues as well as being detected in plasma and urine. Hyal-1 is not membrane bound; however, it requires CD44 for hyaluronidase activity in vivo. It is upregulated in bladder and prostate cancers.20
- Hyal-2 is also expressed in most tissues; however, it is absent from the brain. It breaks down extracellular HA. It possesses a glycosylphosphatidylinositol (GPI) signal sequence, which anchors it to the membrane. Hyal-2 also requires CD44 to be able to degrade HA in vivo,20 It is thought to be an extracellular enzyme that is key for tissue remodeling and cellular migration.10
- Hyal-3 is expressed in brain and several other tissues, but its function has not been determined. It is GPI anchored.20
- Hyal-4 is specific for chondroitin sulfate substrates and is GPI anchored.20
- PH-20 is expressed in sperm and is active during fertilization where it degrades the HA-enriched cumulus of the egg.10,20
Chemical7
HA can also be degraded by non-enzymatic means. The most common conditions that result in shorter chain lengths are:
- Acidic conditions
- Alkaline conditions
- Physical stress (high-speed stirring or critical shearing)
- Sonication
- Free-radical based cleavage (hydroxyl radical can initiate HA degradation by non-specifically cleaving the glycosidic linkage)
Hyaluronan Synthesis
HA is made by enzymes called hyaluronan synthases (HAS). HA is synthesized at the plasma membrane with the polymer being extended from the reducing end, which results in its extrusion from the cell surface. This is atypical for glycosaminoglycans.12 CD44 expressing cells will retain the synthesized HA in the form of a pericellular coating.11 The HA used to produce Glycosil® ha and HyStem® hydrogels is synthesized using the recombinant hasA gene from Streptococcus equisimilis, which is expressed in Bacillus subtilis. The resulting HA is in the 1 MDa range.26
Glycosil® HA vs. Unmodified HA
Unmodified HA is difficult to work with (high viscosity), does not form a stable hydrogel (gel-like solutions can be made at high concentrations, especially with high molecular weight HA, but there is no chemical crosslinking) and HA is rapidly degraded in vivo by endogenous hyaluronidases.2,9
Glycosil® HA is formed by introducing multiple thiol moieties to each HA polymer at the COOH or OH groups.12 These thiol groups can react with each other to form disulfide bonds or they can be covalently reacted with PEGDA to create a hydrogel that is stable for 4-8 weeks in vivo,22-25 but can still be degraded by hyaluronidases.
History of Hyaluronan
1934 – HA was first isolated from the vitreous humor of bovine eyes and was named from hyaloid (= vitreous) and uronic acid
1930-40s – HA was isolated from synovial fluid, skin, umbilical cord, tumors and rooster combs
1951 – Determination of HA chemical structure
1970s – Cartilage proteoglycans were shown to interact specifically with HA
1993 – Discovery and cloning of hyaluronan synthase from Group A Streptococcus
1999 – First purification of active hyaluronan synthase
Present– HA production is now primarily by bacterial fermentation
References
Pour continuer à lire, veuillez vous connecter à votre compte ou en créer un.
Vous n'avez pas de compte ?