Manual Purification with HisTrap FF Crude Kit
HisTrap™ FF crude Kit is designed for rapid and convenient manual purification of histidine-tagged proteins using premade buffers and a syringe. Histidine-tagged proteins can be purified directly from unclarified cell lysates. This saves time because the pretreatment of the sample is minimized.
The kit contains three ready-to-use 1 mL HisTrap™ FF crude columns (containing Ni Sepharose® 6 Fast Flow), buffer concentrates, a 5 mL syringe, and connectors. The kit provides a sufficient volume of buffer concentrates to perform 10 to 12 purifications when operated with a syringe. The special design of the column, together with Ni Sepharose® 6 Fast Flow, provides fast, easy, and reproducible separations in a convenient format. Note that HisTrap™ FF crude columns cannot be opened or refilled.
Direct loading of unclarified cell lysates decreases the total purification time and may increase the possibility of purifying sensitive target proteins with retained activity.

Fig 3.19.HisTrap™ FF crude Kit provides convenient and simple purification of histidine-tagged proteins.
Sample preparation
Refer to Purification using Ni Sepharose® 6 Fast Flow earlier in this chapter for a general procedure for sample preparation. Information specific to HisTrap™ FF crude Kit is included below.
For direct loading of an unclarified sample, it is critical to obtain efficient cell lysis in order to avoid problems with back pressure. Apply the unclarified lysate on the column directly after preparation.
If the sample is viscous, dilute it with binding buffer to prevent it from clogging the column; increase lysis treatment (sonication, homogenization); or add DNase/RNase to reduce the size of nucleic acid fragments.
If the sonicated or homogenized unclarified cell lysate is frozen before use, precipitation and aggregation may increase. Samples with precipitation may be sonicated to reduce clogging of the wells. Note that aging of the sample may reduce yields of the target protein.
The high salt concentration in the buffer stock solution may cause salt crystals to form at low temperature. These crystals will dissolve at room temperature. We therefore recommend that the buffer stock solutions be allowed to reach room temperature before use. The formation of salt crystals that dissolve at room temperature does not affect the performance of the product. The prepared binding and elution buffers can be used in a cold room as well as at room temperature.
Table 3.2 provides the mixing table for 50 mL of buffer. To obtain the imidazole concentration indicated in the first column, mix Phosphate buffer 8× stock solution, 2 M imidazole, and distilled water according to the table. Check pH and adjust to pH 7.4 to 7.6 if necessary. These buffers will contain 20 mM phosphate, 500 mM NaCl, and the concentrations of imidazole indicated. For one purification, 24 mL of the binding buffer and 8 mL of each elution buffer are sufficient.
Ni Sepharose® is compatible with reducing agents. However, we recommend removal of any weakly bound Ni2+ ions before applying buffer/sample that includes reducing agents. This can be accomplished by performing a blank run without reducing agents (see next page). Do not store HisTrap™ FF crude columns with buffers that include reducing agents.
Leakage of Ni2+ from Ni Sepharose® is very low under all normal conditions. For very critical applications, leakage during purification can be even further diminished by performing a blank run before loading sample.
Blank run:
Use binding buffer and elution buffer without reducing agents.
- Wash the column with 5 column volumes of distilled water (to remove the 20% ethanol).
- Wash with 5 column volumes of elution buffer.
- Equilibrate with 10 column volumes of binding buffer.
Standard purification
When high yield is more important than optimal purity, use the following procedure. When optimal purity is required, use the optimizing procedure (next page) instead.
- Using the buffer concentrate provided, prepare 24 mL of binding buffer.
- Using the buffer concentrate provided, prepare 8 mL of elution buffer.
- Fill the syringe with distilled water. Remove the stopper and connect the column to the syringe with the provided Luer connector “drop to drop” to avoid introducing air into the column. (If air becomes trapped in the column, wash it with distilled water until the air disappears.)
- Remove the snap-off end. Wash the column with 5 mL of distilled water.
- Using the syringe, equilibrate the column with 5 to 10 mL of binding buffer.
- Apply the unclarified lysate with the syringe. Collect the flowthrough fraction. A pump (e.g., Peristaltic Pump P-1) is convenient for large volumes (more than 15 mL) using a maximum flow rate of 3 mL/min.
Sample loading at 4°C may increase the viscosity of the sample. An adverse effect of increased sample viscosity is that maximum back pressure for the column is reached at a lower sample volume loading on the column. Large volumes may increase back pressure, making the use of a syringe more difficult. Typical loading volumes of unclarified lysate (highly dependent on specific sample, sample pretreatment, and temperature at sample loading): Up to 100 mL.
- Wash with 10 mL of binding buffer. Collect the wash fraction.
- Elute with 5 mL of elution buffer. Avoid dilution of the eluate by collecting it in 1 mL fractions.
- Check the different fractions for the purified protein (e.g., by SDS-PAGE and/or Western blotting). The purified protein is most likely found in the second and third milliliter of the elution step.
For A280 measurement, use the elution buffer as a blank. If imidazole needs to be removed, use a desalting column (see Chapter 11, Desalting/buffer exchange and concentration). Low-quality imidazole will give a significant background absorbance at 280 nm.
- After elution, regenerate the column by washing it with 10 mL of binding buffer. The column is now ready for a new purification of the same target protein.
The column does not need to be stripped and recharged between each purification if the same protein is going to be purified. Reuse of any purification column depends on the nature of the sample and should only be performed with identical tagged proteins to prevent cross-contamination. For more information on this topic and on cleaning and storage, refer to Appendix 1 (Characteristics of Ni Sepharose, Ni Sepharose® excel, TALON Superflow, and uncharged IMAC Sepharose® products).
Optimizing purification
When a high purity is needed, the following general procedure for stepwise gradient elution should be used. The next time the same protein is to be purified, the number of steps can be reduced to those described under “Standard purification” with the optimal imidazole concentrations selected here.
- Prepare binding buffer and five steps of elution buffer ranging from 40 mM to 500 mM imidazole. Check pH of each after mixing and adjust to pH 7.4 to 7.6 if necessary. Table 3.2.
- Fill the syringe with distilled water. Remove the stopper and connect the column to the syringe with the provided Luer connector “drop to drop” to avoid introducing air into the column. (If air becomes trapped in the column, wash it with distilled water until the air disappears.)
- Remove the snap-off end. Wash the column with 5 mL of distilled water.
- Using the syringe, equilibrate the column with 5 to 10 mL of binding buffer.
- Apply the unclarified lysate with the syringe. Collect the flowthrough fraction. A pump (e.g., Peristaltic Pump P-1) is convenient for large volumes (more than 15 mL) using a maximum flow rate of 3 mL/min.
Note: One mL/min corresponds to approximately 30 drops/min when using a syringe with a HiTrap™ 1 mL column, and 5 mL/min corresponds to approximately 120 drops/min when using a HiTrap™ 5 mL column.
Sample loading at 4°C may increase the viscosity of the sample. An adverse effect of increased sample viscosity is that maximum back pressure for the column is reached at a lower sample volume loading on the column. Large volumes may increase back pressure, making the use of a syringe more difficult. Typical loading volumes of unclarified lysate (highly dependent on specific sample, sample pretreatment, and temperature at sample loading): Up to 100 mL.
- Wash with 10 mL of binding buffer. Collect the wash fraction.
- Start elution with 5 mL of the first elution buffer containing 40 mM imidazole. Avoid dilution by collecting the eluate in 1 mL fractions.
- Proceed with the next imidazole concentration. (For example, elute with 5 mL of elution buffer containing 60 mM imidazole.) Collect the eluate in 1 mL fractions as above.
- Proceed with the buffers of increasing imidazole concentration, as described in steps 6 and 7. The purified protein is most likely found in the second and third fraction of one of the elution steps.
- Check the different fractions for the purified protein (e.g., by SDS-PAGE and/or Western blotting).
For A280 measurements, use the elution buffers as blanks. If imidazole needs to be removed, use a desalting column (see Chapter 11, Desalting/buffer exchange and concentration). Low-quality imidazole will give a significant background absorbance at 280 nm.
- After elution, reequilibrate the column with 10 mL of binding buffer. The column is now ready for a new purification of the same target protein.
The column does not need to be stripped and recharged between each purification if the same protein is going to be purified. Reuse of any purification column depends on the nature of the sample and should only be performed with identical tagged proteins to prevent cross-contamination. For more information on this topic and on cleaning and storage, refer to Appendix 1 (Characteristics of Ni Sepharose, Ni Sepharose® excel, TALON Superflow, and uncharged IMAC Sepharose® products).
The results of the above purification provide information about the optimal binding and elution buffers. The optimal elution buffer is the one that eluted the histidine-tagged protein. The optimal binding (wash) buffer is the one from the step before, with a lower concentration of imidazole. Using the highest possible concentration of imidazole in the binding buffer will give the highest purity of the purified protein. Use these buffers for the next purification of an identical protein.
The concentration of imidazole needed to prevent nonspecific binding of host cell proteins
(without any elution of histidine-tagged protein) is generally more important to determine than the concentration needed for elution. A concentration of 500 mM can be used for elution in most cases.
Application example
Purification using HisTrap™ FF crude Kit
HisTrap™ FF crude Kit includes three 1 mL HisTrap™ FF crude columns, ready-made binding and elution buffer concentrates, connectors, a syringe, and instructions. The kit allows purification in a matter of minutes starting from an unclarified cell lysate using the following procedure:
- Lyse cells containing histidine-tagged protein.
- Prepare buffers by mixing and diluting the concentrates.
- Use the syringe to load unclarified sample to the column, wash, and elute the target protein.
- Check purity by SDS-PAGE.
As Figure 3.20 shows, MBP-(His)6 is effectively purified on the HisTrap™ FF crude 1 mL column using a syringe and the buffers included in HisTrap™ FF crude Kit. In this case, 30 mM imidazole was chosen for the wash and 300 mM imidazole for complete elution.
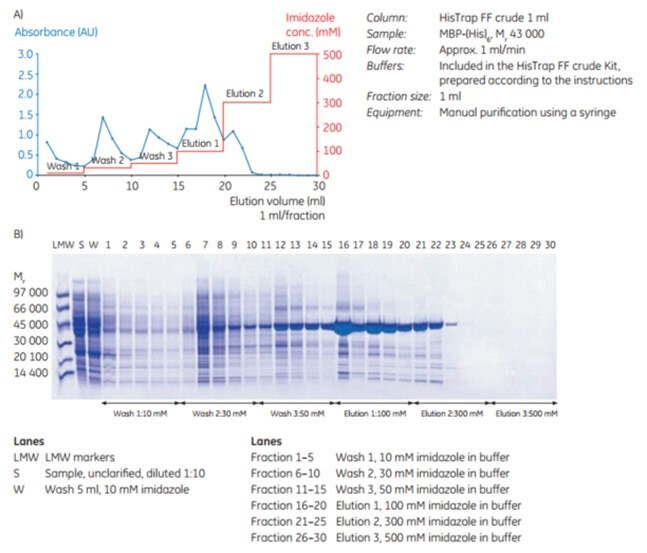
Figure 3.20.(A) Purification of MBP-(His)6 using HisTrap™ FF crude Kit. (B) Native SDS-PAGE (ExcelGel 8–18) of 1 mL fractions from the purification.
Pour continuer à lire, veuillez vous connecter à votre compte ou en créer un.
Vous n'avez pas de compte ?