mRNA Process & Cost Modeling: Optimize Development
David Loong, Head of Emerging Biotech (South Asia & Oceania), Merck, Cory Peterson, Sr. Project Manager, Market Modeling, Strategy Analytics & Intelligence, Merck
Introduction
Owing in part to the success of mRNA-based vaccines developed against the SARS-CoV-2 virus, interest in this modality to mitigate the impact of other infectious diseases and to treat diseases like cancers continues to grow. This interest is also driven by the fact that the production of mRNA is relatively simple compared to other biologics, it is wholly synthetic, and does not require cellular materials.
We developed a cost-model to compare mRNA-based vaccines with other modalities to guide informed decision-making regarding production scale and plant sizing. This article highlights several aspects of the mRNA cost-modeling initiative such as the distribution of costs and cost per dose, and proposes various facility scenarios to meet forecasted mRNA demand.
Cost modeling approach
Our cost-model required definition of the mRNA process flow and made assumptions regarding recovery, titer, and process parameters (Workflow 1). Applying the technique of mass/material balances resulted in anticipated process volumes, which then allowed for the sizing of consumables and equipment, together with the estimation of labor, floor space, and utilities.
Input
- Process flow
- Pricing
- Assumptions
- Process parameters
Model
- Mass/marerial balances
- Sizing for consumables and equipment
- Utilities required
Outputs
- Cost of manufacturing (including details enabling analysis)
- Capital expenses
- Bill of materials
Workflow 1. Cost modeling inputs and outputs.
By factoring in the price of each component, the costs of manufacturing and capital expenses can be derived as outputs. This method also generated a bill of materials for each run.
The utility of cost models and what they cannot tell us
- Cost modeling can be used to show:
- Manufacturing and capital costs within orders of magnitude
- Mass/material balances and associated bills of materials
- The size of consumables and hardware required for various scales
- Time, labor, floorspace and the amount of utilities required
- Cost modeling cannot:
- Predict if the specified process will work from a technical standpoint
- Give absolute costs with high accuracy
- Address elements of uncertainty such as market changes, or account for acknowledged or mystery unknowns
- Predict the transformative impact of future technical innovations
The mRNA process modeled
The mRNA process that was modeled began with plasmid DNA as the in vitro transcription template and ended with the formulated lipid nanoparticle (LNP) product (Figure 1). The process used the Vaccinia capping reagent, with a separate enzymatic capping step. Purification by UF/DF and chromatography was included after each synthesis step, and prior to LNP formulation. Step yields and recoveries were estimated based on literature reports.
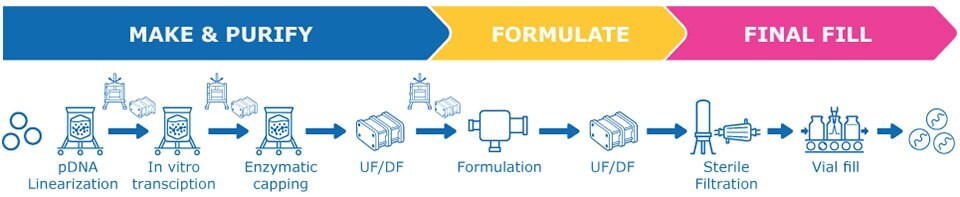
Figure 1.Schematic of mRNA process used in cost model.
Note that a process template for mRNA production has not been established and that this model does not constitute an endorsement or recommendation of any particular process; rather, it is a composite of a possible process based on what is published in the literature.
Co-transcriptional capping and the mRNA process
The mRNA manufacturing field is rapidly advancing, and co-transcriptional capping using the CleanCap® reagent is gaining in popularity due to better overall efficiency.
Overall reagent costs* are ~50% less for the CleanCap® process because poly(A) polymerase, 2’-O-methylfransferase, and associated reagents are not needed. The enzymatic capping process was modeled as one synthetic plus two purification steps with an overall 58% yield. A co-transcriptional capping process using the CleanCap® reagent eliminated these unit operations while maintaining an overall step recovery of 95-98%.
For a given amount of mRNA made, the enzymatic capping process is approximately half as efficient as a co-transcriptional capping process and will be at least 4x more expensive in terms of reagents.
*Note that licensing fees for such advanced reagents were not taken into account for this exercise.
Distribution of costs at various scales at maximum capacity
The maximum possible output of mRNA at various scales of IVT is shown in Table 2. Installed capital costs, cost of goods (CoGs) per dose, and a breakdown of cost categories was determined for the maximum throughput (Figure 2).
Maximum capacity means:
- 2 batches per week
- 40 mfg weeks/year
- No losses due to testing

Figure 2.Distribution of costs per IVT batch size, from 1L – 200L.
The model showed that costs of materials (chemicals and reagents) remained fixed regardless of scale, which aligns with the fact that material costs linearly correlated with the amount of mRNA synthesized. Although the total CoGs per dose decreased with increasing economies of scale, it plateaued at scales of 50 L and higher. Consumables such as filters and single-use bags contributed very little to the overall COGs at scales of 50 L and higher.
The model also revealed that the cost of installed capital rose steeply when the IVT batch size increased beyond bench-top devices (1 L or less) to floor-standing equipment (upwards of 10 L).
The number of batches and batch size needed to meet different forecast demand
In planning the mRNA facility size and footprint, it is useful to first know how much mRNA must be produced in a given year. This annual demand can be achieved either by increasing the size (L) of each batch run, or increasing the number of batches run per year. Three scenarios were modeled to determine demand (Figure 3).

Figure 3.Total IVT volumes needed (L) per forecast demand (g).
Low forecasted annual demand of 1 to 20 g of mRNA
The needs of this scenario can be met using 6 batches of 1 L, or 1 batch of 10 L (Figure 4). Both options will have low facility utilization, resulting in higher installed capital costs.
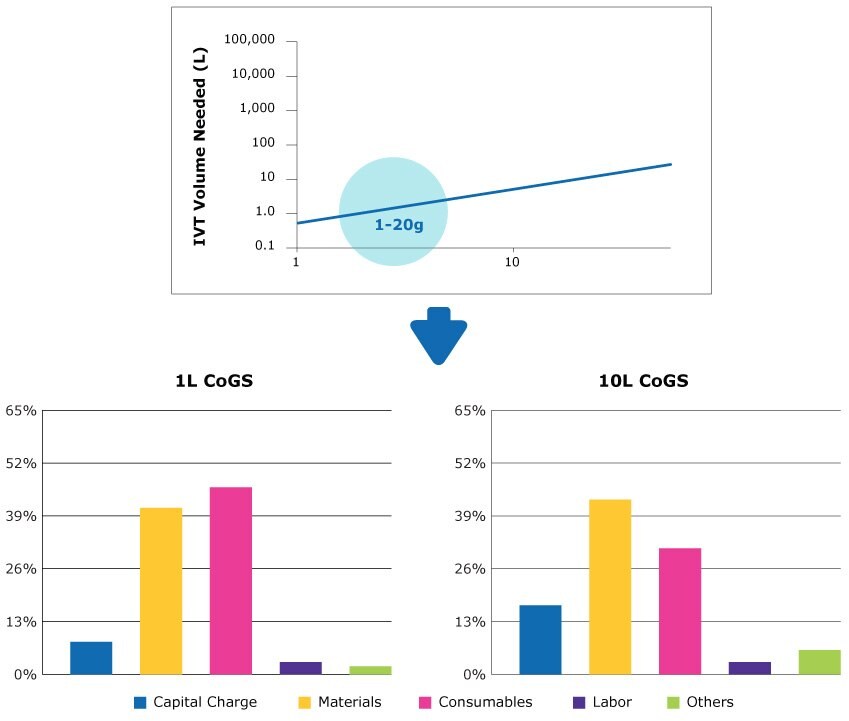
Medium forecasted annual demand of 200 to 400 g of mRNA
For a mid-range forecasted demand, running 2 x 1L lines provides a similar output to a single 10 L line, but with much lower installed capital costs (Figure 5). This scenario might be useful in a multi-product facility with fluctuating demand.
Although annual forecasted demand could also be met by running a single 200 L batch, installed capital would be nearly 3x that of the 1L scale, and is the largest contributor to overall costs. Nevertheless, this scenario might also be well-suited for pandemic preparedness where surges in demand need to be addressed.
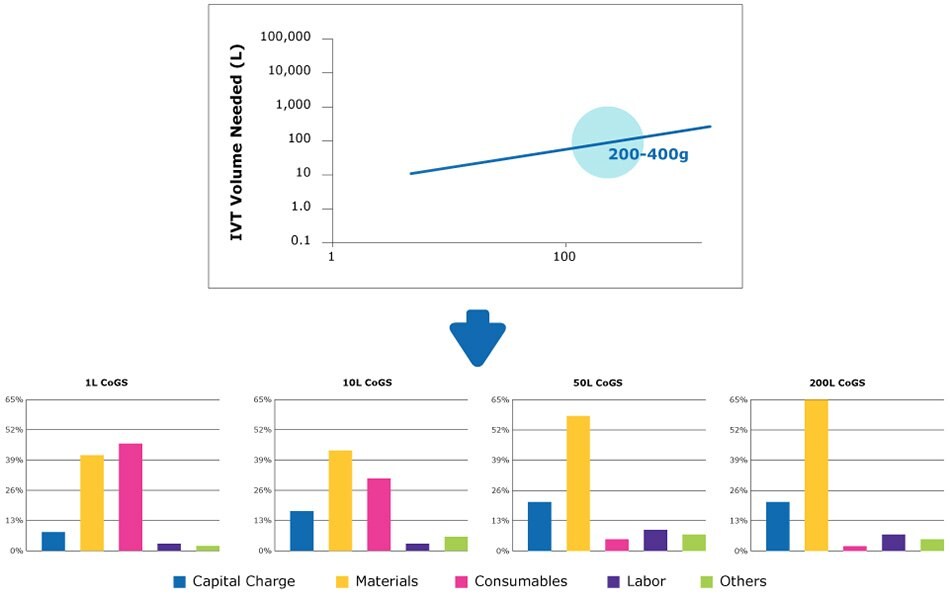
High forecasted annual demand of approximately 1kg of mRNA
For high annual demand, a minimum batch size of 10 L would be required. By running at maximum capacity (2 batches per week, 80 batches per year), capacity utilization can be maximized (Figure 6).
A 50 L batch size and 16 batches per year could be considered to optimize operational flexibility and facility utilization.
Key takeaways and conclusions: Start with the outcome in mind
This article describes a model created to identify cost-drivers for various annual demand for mRNA.
- Once the forecasted demand for mRNA is known, the impact on facility design can be assessed. This will determine the number of lines, batch size and number of batches. Plan your facility for flexibility but optimize for capacity.
- Ensure the process is fully understood and create a process and cost model specific to this approach.
- These cost-modeling scenarios indicate that single-use consumables contribute little to the overall cost-of-goods. However, they enable flexibility and rapid facility turnaround. Single-use technologies form part of a robust contamination control strategy, while enabling facility throughput to be maximized.
- To ensure a cost-effective and high-quality process, partner with a technology provider that has global experience in the manufacturing of multiple vaccine and therapeutic modalities.
Acknowledgements: Dr Julian Mochayedi, Strategic Marketing Manager for mRNA Solutions, Life Science Services; for insightful review.
Discover our Comprehensive Solutions for Your mRNA Manufacturing, and more resources on SigmaAldrich.com/mRNA.
Discover our Comprehensive Solutions for Your Vaccine Manufacturing, and more resources on SigmaAldrich.com/vaccine-manufacturing.
To continue reading please sign in or create an account.
Don't Have An Account?