Magnetic-Plasmonic Hybrid Nanobeads Designed for Imaging and Isolation of Cellular Organelles
Mari Takahashi, Shinya Maenosono
School of Materials Science, Japan Advanced Institute of Science and Technology
Material Matters, 2020, 14.4
- Introduction
- Brief History of MPNBs
- Synthesis and Characterization of MPNBs
- Morphology and Properties of MPNBs
- Imaging and Isolation of Autophagosomes Using MPNBs
- Conclusion
- Acknowledgements
Introduction
Cells take up extracellular solute and proteins/lipids on the plasma membranes by endocytosis and package them into endocytic vesicles. The internalized materials are then either delivered to lysosomes for degradation or recycled back to the plasma membrane for reuse. The endocytosis process is fundamental to many other biological processes, including nutrition uptake, regulation of mitogenic signaling, cell differentiation and locomotion, and immune response. Moreover, some pathogens such as viruses and bacteria exploit endocytosis to invade host cells. It follows that the study of the molecular machinery that regulates endocytosis and characterization of the protein/lipid components of endocytic vesicles is essential for improving our fundamental understanding of cell biology and pathology. Recently, magnetic separation of endocytic vesicles/endosomes has been reported by several groups.1,2 These magnetic separation techniques enable recovery of purified and undamaged target material, and serve as useful alternatives to other techniques such as ultracentrifugation and density-gradient separation.3
Immunomagnetic isolation separates cells and subcellular organelles using magnetic beads coupled with cell-surface or organelle-specific antibodies. In contrast to traditional purification methods using ultracentrifugation, immunomagnetic isolation is rapid and gentle, protecting the integrity of isolated cells and organelles. Magnetic beads have been used to isolate endocytic organelles—such as early and late endosomes—through endocytosis of beads into the lumen of organelles. We have previously fabricated ultrasmall magnetic beads with an Ag/FeCo/ Ag core/shell/shell structure.4 Although these magnetic probes have a magnetically inert Ag core (mean size 10 nm), these beads still facilitate separation. Further, the Ag core enables visualization of the bead using a microscope. The intrinsic optical properties and localized surface plasmon resonance of Au and Ag metallic nanoparticles has led to their use in cell imaging.5,6 The combination of these materials enables magnetic beads with plasmonic properties; hereafter they are referred to as magneticplasmonic hybrid nanobeads (MPNBs).
Brief History of MPNBs
The superparamagnetic properties of magnetic nanoparticles enable their use in applications such as magnetic hyperthermia, magnetic resonance imaging, contrast agents, magnetic separation, and magnetic drug delivery. In addition, the plasmonic properties of metal nanoparticles enable technologies such as sensing, imaging and photothermal therapy. Therefore, combining these properties into a single nanostructure offers more highly diverse applications than when applying either superparamagnetic or plasmonic properties alone. Several types of MPNBs have been reported including Au-Fe3O4 heterostructured MPNBs,7 Co/Au core/shell MPNBs,8 and FePt-Au heterostructured MPNBs.9 Table 1 overviews MPNBs with various functionalities stemming from individual magnetic and plasmonic components.
Assorted liquid-phase synthetic methods have been investigated to obtain different types of MPNBs such as alloy, core/shell, core/satellite, or heterodumbbell structures with different combinations of magnetic and plasmonic materials. In the following section typical wet-chemical synthesis methods for MPNBs are reviewed.
Aqueous reduction method
In the aqueous reduction method, MPNBs are typically synthesized by sequential reductions of plasmonic and magnetic precursors with a reducing agent in an aqueous solvent. Santhi et al. synthesized Ag-Ni alloy MPNBs by adding a reducing agent to aqueous solution containing Ag and Ni precursors.32 Wang et al. reported a composition-tunable synthesis method of Co/Ag core/shell MPNBs by injecting Ag precursors into aqueous solution containing Co precursors and reducing agents.33 Kostevšek et al. reported FePt/SiO2/Au core/shell/shell MPNBs using a combination of three methods: the reduction method for FePt core formation, the sol-gel method for SiO2 shell formation and the seed-mediated growth method for Au shell formation.34
Heat-up method
Among organic synthesis methods, the heat-up method is facile and reliable for synthesizing uniform MPNBs in a controlled fashion. Typically, metal precursors and stabilizers are dissolved in organic solvent with or without reducing agents. Then, the reaction mixture is heated to high temperature to promote the reduction/decomposition reaction. By precisely controlling the reaction temperature, various types of uniform MPNBs can be synthesized. For example, Yu et al. synthesized dumbbelllike Au-Fe3O4 MPNBs by using the heat-up method.35 Peng and coworkers synthesized Au/Ni and Ag/Ni core/shell MPNBs using the heat-up method in combination with a sequential reduction of precursors.36,37 As an example of a seed-mediated growth method, which is a form of the heat-up method, Wang et al. synthesized Fe3O4/Au core/shell MPNBs using a modified polyol reduction (polyol method) of Au precursors onto preformed iron oxide nanoparticles.38
Hot injection method
Hot injection is another organic synthesis method used to obtain well-defined uniform MPNBs. Typically, a stock solution of metal precursor(s) is injected into organic solvent containing stabilizers at high temperature. This method enables decoupling nucleation and growth of nanoparticles; thus, wider varieties of uniform MPNBs can be synthesized. Bao et al. synthesized Co/Au core/shell MPNBs by a two-step injection method in which Co cores were formed, followed by Au shell formation onto the cores.39 Peng et al. synthesized dumbbell-like Ag-Fe3O4 MPNBs by injecting Ag precursors into a solution containing Fe/FexOy core/shell magnetic nanoparticles.40 Mohan et al. synthesized Au100-x-yFexPty ternary alloy MPNBs by injecting Au and Fe precursors into a reaction solution containing Pt precursors.41
Solvothermal method
The solvothermal method is another important method for preparing MPNBs in which chemicals are reacted under extreme conditions such as middle-high temperature (100−500 °C) and pressure (1−10,000 atm). In a typical solvothermal method, the reaction mixture containing metal precursors and stabilizers are heated with a Teflon-lined stainless-steel autoclave. This method enables the use of the enhanced solubility of metal precursors by exceeding the boiling point of a solvent under an elevated pressure. Zhai et al. reported a size-tuned solvothermal synthesis of Ag/Fe3O4 core/satellite nanostructures.42 Shan et al. also synthesized Ag-Fe3O4 nanocomposites using the solvothermal method.43 In their synthesis, they tuned magnetic and plasmonic properties by changing the input molar ratio of Ag and Fe precursors.
Synthesis and Characterization of MPNBs
Ag/FeCo/Ag core/shell/shell MPNBs were synthesized by combining the hot injection and polyol methods.4,44 First, silver nitrate (AgNO3) (204390), 1,2-hexadecanediol (213748), oleic acid (O1008), oleylamine (OLA) (O7805), and tetraethylene glycol (110175) were combined. The reaction mixture was degassed with Argon (295000) for 5 minutes at room temperature with vigorous stirring, then heated to 100 °C for 10 minutes to remove volatile substances. The temperature was increased to 170 °C and a solution of iron(III) acetylacetonate [Fe(acac)3] (517003) and cobalt(II) acetylacetonate [Co(acac)2] (227129) in OLA and toluene (244511) was added. The temperature was increased to 250 °C, and a solution of AgNO3 in OLA and toluene was added to form the Ag outer shell. The temperature was reduced to 230 °C for 15 min and then to room temperature. Acetone (179124) was added and the MPNBs were centrifuged and subsequently redispersed in hexane (296090). All chemicals were purchased from Sigma-Aldrich and used as received. The MPNBs were then modified with thiolated ε-poly-l-lysine (PLLSH). The MPNBs were characterized using transmission electron microscopy (TEM), high-resolution TEM (HRTEM), scanning TEM equipped with a high-angle annular dark-field (STEMHAADF) detector, energy-dispersive X-ray spectroscopy (EDS) elemental mapping, X-ray photoelectron spectroscopy (XPS), X-ray diffraction (XRD), inductively coupled plasma optical emission spectroscopy (ICP-OES), superconducting quantum interference device (SQUID) magnetometry, and ultravioletvisible spectroscopy (UV-vis).
Morphology and Properties of MPNBs
Figure 1 shows TEM and STEM images of as-synthesized Ag/FeCo/Ag MPNBs, these MPNBs were spherical and showed a distinct core-shell structure. The average diameter of MPNBs was calculated to be ~15 nm.4,44-47 It is evident from these images that the MPNBs have a distinct Ag core and FeCo shell. Figure 1H shows the EDS line profile (yellow line in Figure 1G). The Ag signal clearly increased at the edge of the MPNBs confirming the presence of a thin Ag outer shell.

Figure 1.A) TEM, B) HRTEM, and C) STEM-HAADF images of MPNBs. D) Ag L, E) Fe K, F) Co K, and G) merged images of EDS elemental mapping images for MPNBs. H) EDS line profile along the yellow line in G). Reprinted with permission from reference 47, copyright 2017 American Chemical Society.
Figure 2 shows Fe 2p and Co 2p XPS spectra for the MPNBs.45 The atomic ratio between Fe and Co was determined to be Fe:Co = 67:33. In contrast, the Fe:Co composition measured by ICP-OES was near equiatomic. This discrepancy is attributed to a composition gradient within the FeCo shell, with Co being rich near the Ag core, while Fe is rich on the surface of the FeCo shell. The relative oxidation state proportions of each species were Fe0/Fe2+/Fe3+ = 15:56:29 and Co0/Co2+/Co3+ = 23:47:30. X-ray diffraction (XRD) analysis of the MPNBs revealed that FeCo and CoxFe1-xO phases are simultaneously present.45 Considering all of the data together, it can be said that the surface of the FeCo shell is likely to oxidize to form the CoxFe1-xO cobaltwüstite layer. In order to confirm this supposition, the magnetic properties of the MPNBs were examined using a superconducting quantum interference device (SQUID) magnetometer.
Figure 3 shows magnetization (M-H) curves recorded at T = 5 K, after zero-field cooling (ZFC) and field cooling (FC) in a 1 T magnetic field. As shown in Figure 3, the hysteresis loop after the FC procedure was shifted along the field axis in the negative direction to the cooling field, indicating the presence of exchange bias. This result also suggests the existence of an interface between ferromagnetic (FeCo) layer and antiferromagnetic (CoxFe1-xO) layer within the shell.45

Figure 2.A) Fe 2p and B) Co 2p XPS spectra for MPNBs. Black, yellow, blue, and red curves represent raw data, Shirley background, deconvolved peaks, and the sum of all components, respectively. Reprinted with permission from reference 45, copyright 2016 Elsevier.
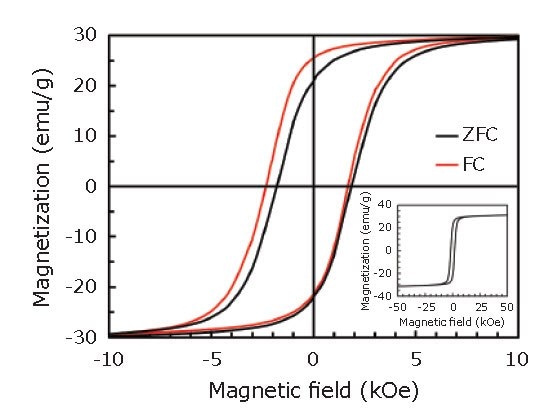
Figure 3.M-H curves in the range of -10 to 10 kOe recorded at T = 5 K, after ZFC (black) and FC (red) procedures. The inset shows the entire M-H curve after the ZFC procedure. Reprinted with permission from reference 45, copyright 2016 Elsevier.
Based on the STEM, XPS, XRD, and SQUID analyses, we concluded that the detailed structure of a single MPNB is as shown in Figure 4. We also note that the magneto-optical properties of the MPNBs were found to be modified with an enhancement of the Faraday rotation and ellipticity of 5× compared with the pure FeCo nanoparticles.48 The modifications are mainly due to the plasmonic core in the hybrid structure and the strong coupling between the magnetic and plasmonic materials. Such strong coupling was achieved owing to the bead morphology (i.e. the plasmonic structure at the core).
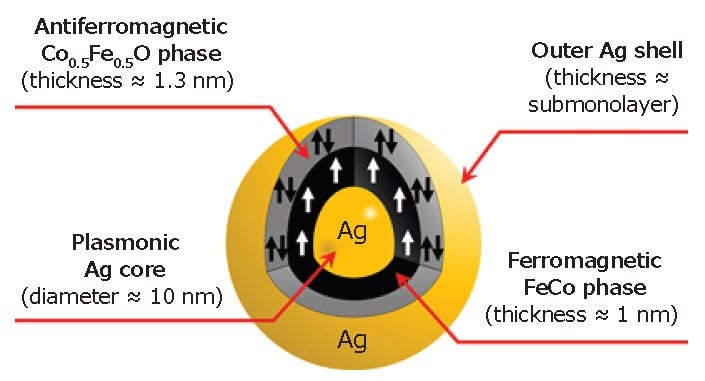
Figure 4.Illustration of a single MPNB showing its internal structure.
We elucidated the formation mechanism of Ag/FeCo/Ag MPNBs was a combination of two well known phenomena: (1) competition between size focusing and Ostwald ripening and (2) size-dependent catalysis of Ag nanoparticles. The injection of Ag precursor causes size focusing of the Ag cores such that the monodispersed cores are larger than the critical size. Consequently, electrons are transferred from the polyol to the Ag cores and the Ag cores to the Co or Fe ions, and then the FeCo shells are formed uniformly on the Ag cores. During FeCo shell formation, Ag atoms are simultaneously incorporated into the FeCo shell, and the surface segregation of Ag takes place spontaneously, resulting in formation of an Ag outer shell.44
Imaging and Isolation of Autophagosomes Using MPNBs
Autophagosomes were chosen as target organelles. It has been reported that micrometer-sized polystyrene beads lipofected into mammalian cells are delivered into early endosomes causing damage and inducing xenophagy, whereby damaged endosomes are engulfed by autophagosomes.49,50 Although autophagosomes can engulf micrometer-sized beads,49 we used MPNBs to demonstrate the ability of MPNBs to magnetically isolate subcellular organelles.
PLL-SH-modified MPNBs were transfected into COS-1 cells by lipofection.47 Cells were then fixed, stained for Vps26 (early endosomal marker protein) or LC3 (autophagosomal marker protein), and examined by confocal laser scanning microscopy (CLSM). Thirty minutes after transfection, the MPNBs were partially co-localized with Vps26, but not with LC3 (Figure 5A–C). One hour after lipofection, some MPNBpositive structures surrounded by LC3 emerged (Figure 5D). These structures were mostly evident 2 and 4 h after lipofection (Figures 5E and F). This indicated that the MPNBs were first delivered into early endosomes, and then targeted to autophagosomes. Imaging of MPNBs was achieved using plasmon scattering.
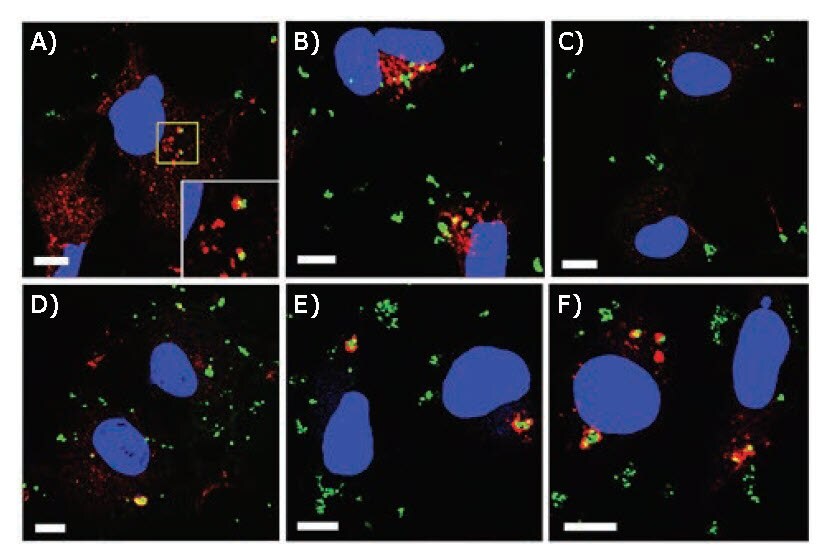
Figure 5.CLSM images of MPNB-transfected COS-1 cells after incubation for A) 30 min and B) 1 h for Vps26 staining, and C) 30 min, D) 1 h, E) 2 h, and F) 4 h for LC3 staining. Blue and green represent nuclei and MPNBs, respectively. Red represents Vps26 (A,B) or LC3 (C–F). Scale bars correspond to 10 μm. The inset shown at the bottom right of (A) is a magnified view of the region enclosed by the yellow line in (A). Reprinted with permission from reference 47, copyright 2017 American Chemical Society.
The MPNB lipofected cells were detached from the culture dish and homogenized. The resultant cell lysates were magnetically separated using an autoMACS Pro Separator (Miltenyi Biotec, Germany). The magnetically separated fraction (MS) was eluted from the column, spun briefly, and resuspended in phosphate buffered saline (PBS) (P4244) (Figure 6A). The cell lysate and MS were subjected to SDS-PAGE and blotted for LC3, transferrin receptor (TfnR, an endosomal protein), lysosomal-associate membrane-2 (LAMP2, a lysosomal protein), and glyceraldehyde 3-phosphate dehydrogenase (GAPDH, a cytosolic protein). As shown in Figure 6B, at time 0, no proteins of interest were detected in the MS. After 15 min, LC3-II (a membrane-bound form of LC3), tumor necrosis factor receptor (TfnR), and LAMP2, but not GAPDH, were detected in the MS. The levels of LC3-II and LAMP2 increased up to 8 h, whereas that of TfnR plateaued after 2 h. Throughout the separation experiments, no GAPDH or LC3-I (a cytosolic form of LC3) was detected in the MS. These results indicated that at early time points (0.5<t<2 h, (t is incubation time)) mainly autophagosomes were isolated, and in contrast, at later time points (t>2 h), autophagolysosomes were isolated.47
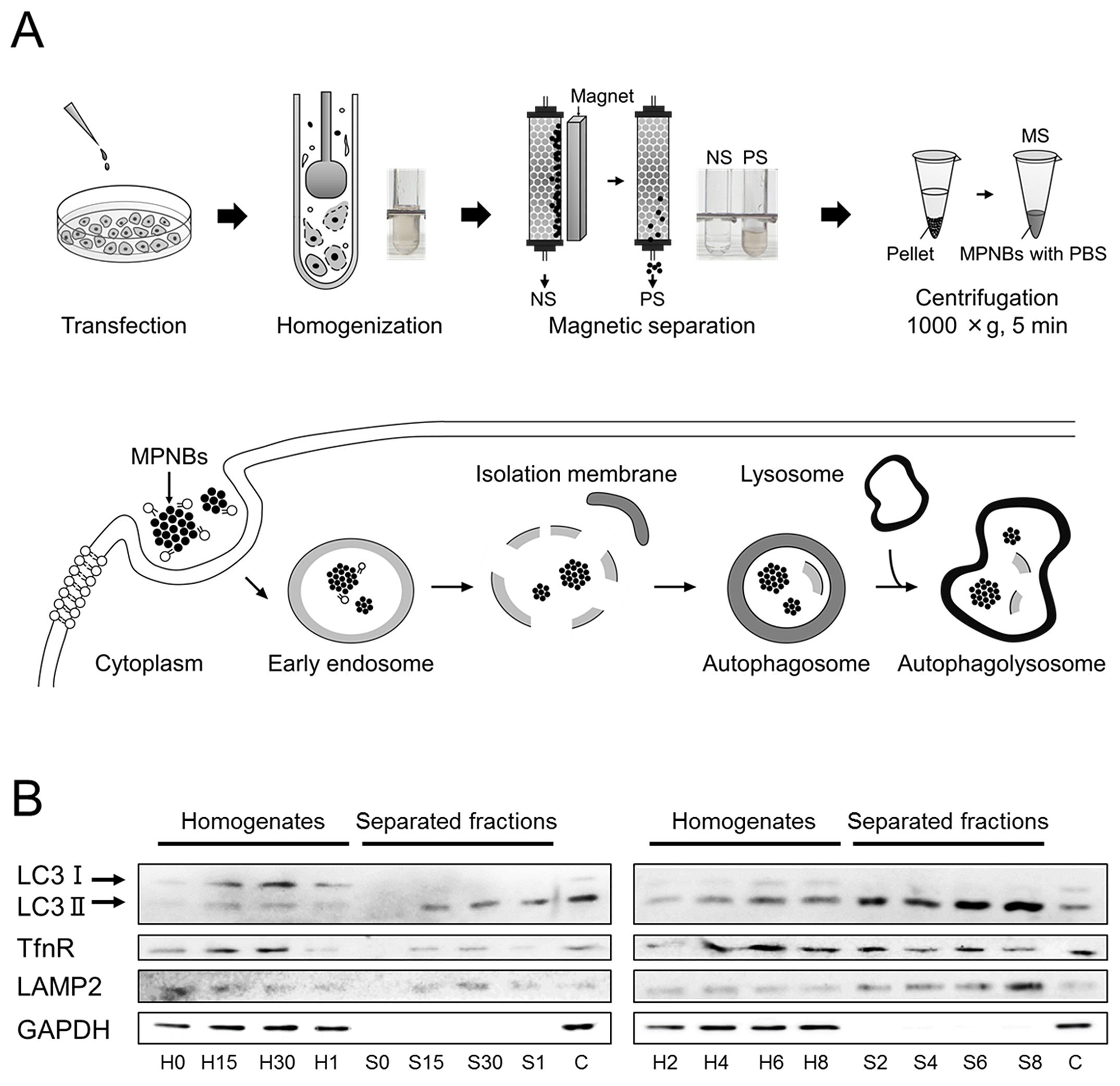
Figure 6.Magnetic separation scheme and western blotting results. A) Schematic illustrations of (top) the magnetic separation protocol and (bottom) the change in the localization of hybrid nanoparticles in a cell. B) The incubation time dependence of the presence of marker proteins in the homogenate (designated H) and the separated fractions (MS, designated S). C is a control in which the transfection reagent was present but nanoparticles were absent. The number added after the initial of the sample (H or S) denotes the incubation time, that is, 0, 15, and 30 correspond to 0, 15, and 30 min incubations, respectively, while 1, 2, 4, 6, and 8 correspond to 1, 2, 4, 6, and 8 h incubations, respectively. Reprinted with permission from reference 47, copyright 2017 American Chemical Society.
Conclusion
Monodispersed Ag/FeCo/Ag core/shell/shell MPNBs were synthesized and transfected into COS-1 cells by lipofection.
After incubation the localization of MPNBs in cells was visualized by confocal laser scanning microscopy (CLSM) using
the plasmon scattering of the Ag cores. The MPNBs reached early endosomes within 30 min, and subsequently became positive for LC3. Autophagosomes containing MPNBs were then successfully isolated by magnetic separation. This process took ~30 min after cell lysis, providing a rapid method for isolating autophagosomes. The ability of MPNBs to magnetically isolate cellular organelles was clearly demonstrated and is promising for isolation of other organelles such as endosome-related organelles, the trans-Golgi network, and clathrin-coated vesicles. By enabling on-demand isolation of cellular organelles, developing MPNBs to identify proteins on/in the organelles, and analyzing the function of these proteins, it will become possible to offer new insights in both biology and clinical medicine.
Acknowledgements
This work was supported by a Grant-in-Aid for Scientific Research, Grant nos. 26600053 and 15J10127. We are indebted to Prof. Tomohiko Taguchi and Assist. Prof. Kojiro Mukai (Graduate School of Life Sciences, Tohoku University, Japan) for their longtime collaboration on cell biology issues. We thank Prof. Masahiro Takakura (Department of Obstetrics and Gynecology, Kanazawa Medical University, Japan) and Dr. Takeo Matsumoto (Graduate School of Medical Sciences, Kanazawa University, Japan) for their assistance in magnetic separation experiments. We also thank Prof. Kazuaki Matsumura, Prof. Yuichi Hiratsuka, Ms. Keiko Kawamoto, and Ms. Chiharu Tatsumi of Japan Advanced Institute of Science and Technology (JAIST) for their assistance in cell experiments. We are grateful to Assist. Prof. Shigetaka Nakamura and Prof. Kenzo Fujimoto of JAIST for their support in performing chemiluminescence detection of western blots. We are also obliged to Prof. Satoshi Waguri (Department of Anatomy and Histology, Fukushima Medical University, Japan) for his valuable comments on TEM images of cell organelles, and Dr. Alberto López-Ortega (Department of Applied Physics, University of Castilla-La Mancha, Spain) and Prof. Paolo Vavassori (CIC nanoGUNE, Spain) for collaborative research on magneto-optical effects of the MPNBs. We are also grateful to Dr. Priyank Mohan (JAIST), Dr. Derrick Mott (IMRAM, Tohoku University, Japan), and Mr. Koichi Higashimine (JAIST) for their support over the years.
To continue reading please sign in or create an account.
Don't Have An Account?