What Are Biomaterials?
Biocompatible Polymers
Biodegradable Polymers
Crosslinkers
Biocompatible Inorganic Materials
Supramolecular Chemistry
Introduction
During the last two decades, significant advances have been made in the development of biocompatible and biodegradable materials for biomedical applications, and in the case of the latter category, industrial applications, as well. In the biomedical field, the goal is to develop and characterize artificial materials or, in other words, "spare parts"1 for use in the human body to measure, restore, and improve physiologic function, and enhance survival and quality of life. Typically, inorganic (metals, ceramics, and glasses) and polymeric (synthetic and natural) materials have been used for such items as artificial heart-valves, (polymeric or carbon-based), synthetic blood-vessels, artificial hips (metallic or ceramic), medical adhesives, sutures, dental composites, and polymers for controlled slow drug delivery. The development of new biocompatible materials includes considerations that go beyond nontoxicity to bioactivity as it relates to interacting with and, in time, being integrated into the biological environment as well as other tailored properties depending on the specific "in vivo" application.2
The parallel field of "biomimetics" may be described as the "abstraction of good design from nature" or, plainly put, the "stealing of ideas from nature". The goal is to make materials for non-biological uses under inspiration from the natural world by combining them with manmade, non-biological devices or processes. This is fast becoming a new research frontier.
Biocompatible Polymers
One area of intense research activity has been the use of biocompatible polymers for controlled drug delivery. It has evolved from the need for prolonged and better control of drug administration. The goal of the controlled release devices is to maintain the drug in the desired therapeutic range with just a single dose. Localized delivery of the drug to a particular body compartment lowers the systemic drug level, reduces the need for follow-up care, preserves medications that are rapidly destroyed by the body, and increases patient comfort and/or improves compliance. In general, release rates are determined by the design of the system and are nearly independent of environmental conditions.3
A convenient classification of controlled-release systems is based on the mechanism that controls the release of the substance in question. The most common mechanism is diffusion. Two types of diffusion-controlled systems have been developed; the first is a reservoir device in which the bioactive agent (drug) forms a core surrounded by an inert diffusion barrier (Figure 1). These systems include membranes, capsules, microcapsules, liposomes, and hollow fibers. The second type is a monolithic device in which the active agent is dispersed or dissolved in an inert polymer (Figure 2). As in reservoir systems, drug diffusion through the polymer matrix is the rate-limiting step, and release rates are determined by the choice of polymer and its consequent effect on the diffusion and partition coefficient of the drug to be released.3,4
In chemically controlled systems, chemical control can be achieved using bioerodible or pendant chains. The rationale for using bioerodible (or biodegradable) systems is that the bioerodible devices are eventually absorbed by the body and thus need not be removed surgically. Polymer bioerosion can be defined as the conversion of a material that is insoluble in water into one that is water-soluble. In a bioerodible system the drug is ideally distributed uniformly throughout a polymer in the same way as in monolithic systems. As the polymer surrounding the drug is eroded, the drug escapes (Figure 3). In a pendant chain system, the drug is covalently bound to the polymer and is released by bond scission owing to water or enzymes.5,6 In solvent-activated controlled systems, the active agent is dissolved or dispersed within a polymeric matrix and is not able to diffuse through that matrix. In one type of solvent-controlled system, as the environmental fluid (e.g., water) penetrates the matrix, the polymer swells and its glass transition temperature is lowered below the environmental (host) temperture.7 Thus, the swollen polymer is in a rubbery state and allows the drug contained within to diffuse through the encapsulant.
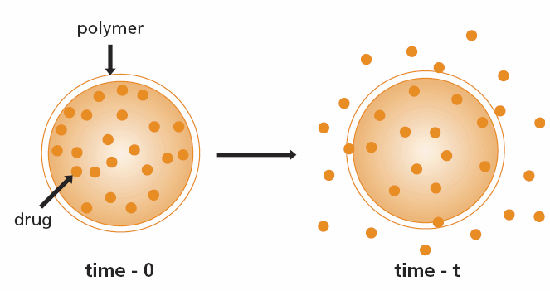
Figure 1.Schematic representation of reservoir diffusion controlled drug delivery device.
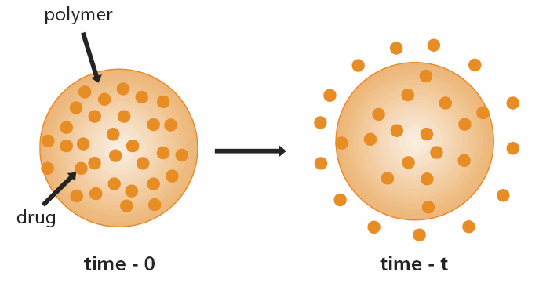
Figure 2.Schematic representation of monolithic (matrix) diffusion controlled drug delivery device.
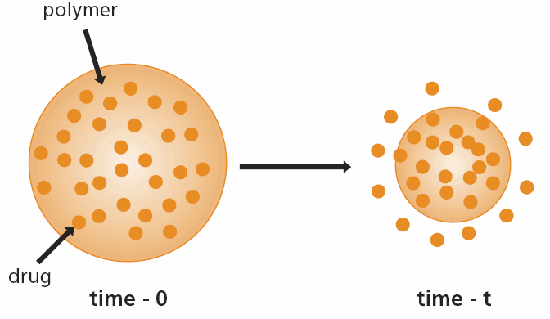
Figure 3.Schematic representation of biodegradable (bioerodible) drug delivery device.
Biodegradable Polymers
A variety of natural, synthetic, and biosynthetic polymers are bio- and environmentally degradable. A polymer based on the C-C backbone tends to be nonbiodegradable, whereas heteroatom-containing polymer backbones confer biodegradability. Biodegradability can therefore be engineered into polymers by the judicious addition of chemical linkages such as anhydride, ester, or amide bonds, among others. Figure 4 provides a schematic representation of the types of polymer degradation. The mechanism for degradation is by hydrolysis or enzymatic cleavage resulting in a scission of the polymer backbone. Macroorganisms can eat and, sometimes, digest polymers, and also initiate a mechanical, chemical, or enzymatic aging.8
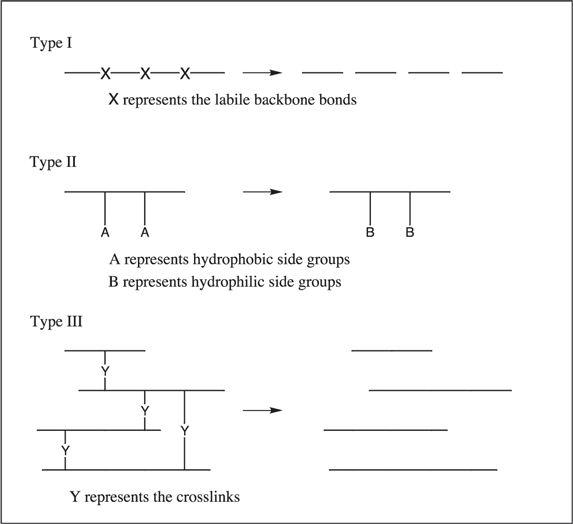
Figure 4.Schematic representation of the types of polymer degradation.9
Biodegradable polymers with hydrolyzable chemical bonds are being researched extensively for biomedical, pharmaceutical, agricultural, and packaging applications.10 In order to be used in medical devices and controlled-drug-release applications, the biodegradable polymer must be biocompatible and meet other criteria to be qualified as a biomaterial-processable, sterilizable, and capable of controlled stability or degradation in response to biological conditions.11 The degradation products often define the biocompatibility of a polymer, not necessarily the polymer itself. Poly(esters) based on polylactide (PLA), polyglycolide (PGA), polycaprolactone (PCL), and their copolymers have been extensively employed as biomaterials.12,13 Degradation of these materials yields the corresponding hydroxy acids, making them safe for in vivo use.
Other bio/environmentally degradable polymers include poly(hydroxyalkanoate)s of the PHB-PHV class, additional poly(ester)s, and natural polymers, particularly, modified poly(saccharide)s, e.g., starch, cellulose, and chitosan.
Chitosan is a technologically important biomaterial. Chitin is the second most abundant natural polymer in the world after cellulose. Upon deacetylation, it yields the novel biomaterial Chitosan, which upon further hydrolysis yields an extremely low molecular weight oligosaccharide (Scheme 1). Chitosan possesses a wide range of useful properties. Specifically, it is a biocompatible, antibacterial and environmentally friendly polyelectrolyte, thus lending itself to a variety of applications14 including water treatment, chromatography, additives for cosmetics, textile treatment for antimicrobial activity,15 novel fibers for textiles, photographic papers, biodegradable films,16 biomedical devices, and microcapsule implants for controlled release in drug delivery.17-19
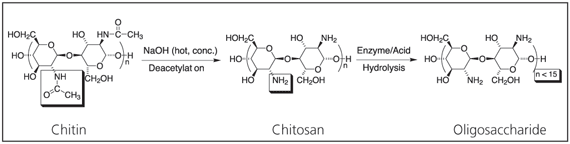
Scheme 1.Deacetylation of chitin to form chitosan and hydrolysis to form oligosaccharide.
Poly(ethylene glycol)s first appeared in the U.S. Pharmacopoeia in 1950. Since then they have been used increasingly for a variety of pharmaceutical applications.
Multiblock copolymers of poly(ethylene oxide) (PEO) and poly(butylene terephthalate) (PBT) are also under development as prosthetic devices and artificial skin and as scaffolds for tissue engineering.20 These materials are subject to both hydrolysis (via ester bonds) and oxidation (via ether bonds). Degradation rate is influenced by PEO molecular weight and content. Additionally, the copolymer with the highest water uptake degrades most rapidly.
A widely used nondegradable polymer is ethylene-vinyl acetate copolymer. This copolymer displays excellent biocompatibility, physical stability, biological inertness, and processability. In drug delivery application these copolymers usually contain 30-50 weight percent vinyl acetate. Ethylene-vinyl acetate copolymer membrane acts as the rate-limiting barrier for the diffusion of the drug. In the Type II class of degradable polymers, the conversion of the hydrophobic substituents to hydrophilic side groups is a first step in the degradation process. A team of researchers has addressed the problem of fabricating open-pore, biodegradable polymer scaffolds for cell seeding or other tissue engineering applications.21 The material selected was the tyrosine-derived polycarbonate poly(DTE-co-DT carbonate), in which the pendant group via the tyrosine, an amino acid, is either an ethyl ester (DTE) or free carboxylate (DT). Through alteration of the ratio of DTE to DT, the material's hydrophobic/hydrophilic balance and rate of in vivo degradation can be manipulated. It was shown that, as DT content increases, pore size decreases, the polymers become more hydrophilic and anionic, and cells attach more readily.
Water-swellable polymer networks may function as hydrogels at one end or as superabsorbers at the other extreme. Hydrogels are characterized by the pronounced affinity of their chemical structures for aqueous solutions in which they swell rather than dissolve. Such polymeric networks may range from being mildly absorbing, typically retaining 30 wt. % of water within their structure, to superabsorbing, where they retain many times their weight of aqueous fluids. Several synthetic strategies22 have been proposed to prepare absorbent polymers including:
- Polyelectrolyte(s) subjected to covalent cross-linking23
- Sssociative polymers consisting of hydrophilic and hydrophobic components ("effective" cross-links through hydrogen bonding)24-26
- Physically interpenetrating polymer networks yielding absorbent polymers of high mechanical strength.27
Clearly, these strategies are not mutually exclusive, and efforts have focused on tailoring composite gels which are critically reliant on the balance between polymer-polymer and polymer-solvent interactions under various stimuli including changes in temperature, pH, ionic strength, solvent, concentration, pressure, stress, light intensity, and electric or magnetic fields.28,29 Such stimuli-responsive polymers, the so-called smart gels, continue to be the subject of extensive investigation for applications in diverse fields. These applications range from biomedical (controlled drug release, ocular devices, and biomimetics),30-32 agricultural (soil additive to conserve water, plant root coating to increase water availability, and seed coating to increase germination rates), and personal care (diapers and adult hygiene products),23,33 to industrial (thickener, gelling agent, cable wrap, specialty packaging, tack reduction for natural rubber, and fine coal dewatering).34-37
Absorbent polymers may be of synthetic (petrochemical) origin where the effects of morphology and porosity affects the absorbent properties.38 Sigma-Aldrich also offers an extensive selection of polymers of natural (starches, etc.) and semisynthetic (cellulose ethers, etc.) origins for use in the synthesis of multicomponent hydrogels.39 To aid in designing your application-specific hydrogel, Sigma-Aldrich offers over 1,500 monomers and a wide selection of cross-linking agents.
Crosslinkers
Crosslinking is the formation of chemical links between molecular chains to form a three-dimensional network of connected molecules. The vulcanization of rubber using elemental sulfur is an example of crosslinking, converting raw rubber from a weak plastic to a highly resilient elastomer. The strategy of covalent crosslinking is used in several other technologies of commercial and scientific interest to control and enhance the properties of the resulting polymer system or interface, such as thermosets and coatings.40-42
Crosslinking has been employed in the synthesis of ion-exchange resins43 and stimuli-responsive hydrogels44 made from polymer molecules containing polar groups. As polyelectrolytes, hydrogels are inherently water soluble. To make them insoluble, they are chemically crosslinked during manufacture or by a second reaction following that of polymerization of the starting monomers. The degree of crosslinking, quantified in terms of the crosslink density, together with the details of the molecular structure, have a profound impact on the swelling characteristics of the crosslinked system. In Figure 4, the loss in crosslinking in response to specific biological conditions results in Type III polymer degradation.
Biocompatible Inorganic Materials
Ceramics may call to mind such objects as teapots and tile floors. There are many additional applications of technical interest, and increasingly, in the biomedical arena. Bioceramics can have structural functions as joint or tissue replacements, can be used as coatings to improve the biocompatibility of metal implants, and can function as resorbable lattices which provide temporary structures and a framework that is dissolved, replaced as the body rebuilds tissue. The thermal and chemical stability of ceramics, their high strength, wear resistance and durability all contribute to making ceramics good candidate materials for surgical implants. Some ceramics even feature drug-delivery capability.
Materials for surgical implants and medical devices must, before all else, be non-toxic. Bioceramics meet that test, and can be, in addition bioinert, that is, not interactive with biological systems; bioactive, that is, durable materials that can undergo interfacial interactions with surrounding tissues; biodegradable, soluble, or resorbable (eventually replaced or incorporated into tissue). Sugars and proteins can bind to some ceramics. Blood vessels, for example, can penetrate some ceramic prosthetics and bone material can eventually begin to replace them.45-47
Due to their strength, flexibility, and biocompatibility, titanium alloys are often used for joint and bone implants. Chromium and cobalt alloys, as well as stainless steel are also, though somewhat less commonly, used for bone implants for similar reasons.
Shape-memory alloys have biomedical applications in procedures such as angioplasty where they can prevent blood vessels from becoming reblocked.48
More recent work has focused on nanocrystalline titanium powders for bone implants. These ultrafine-grained materials utilize the biocompatibility of titanium but are approximately 10 times stronger than conventional titanium implants. It is therefore now possible to use hollow hip implants that more closely match natural bone.49
The perfect material for medical applications would not only be biocompatible, but also have physical properties similar to those of the tissue or other biological system being replaced or repaired. For example, ceramic coatings that mimic the texture and appearance of natural teeth coated over metal supports provide a primary tool for prosthodonic tooth replacement.50
Ceramics, though they include good chemical and corrosion-resistant properties, are notoriously brittle. Researchers therefore have sought ways of combining desirable ceramics with other materials to tailor properties such as strength and elasticity to meet system requirements. Composites, functionally gradient materials, and coatings have been studied to optimize material choices. Ceramic coated, biocompatible metals seem to offer an excellent compromise between the strength and flexibility of metals and the ability of ceramics to be incorporated into biological systems.
Much work has been devoted to the interfacial reactions of biological systems with hydroxyapatite, a ceramic with chemical structure very similar to the hard structure of bone. Hydroxyapatite is used as a coating for metal surgical implants (most often made of titanium and its alloys, or stainless steels), and recent studies have examined the possibility of its use in composite form, in materials that combine polymers with ceramic or metal/ceramic combinations. Considerable research has been performed on methods of coating application and in-situ synthesis of apatites, and the implications for ceramic properties and microstructure.
Ceramics in a number of forms and compositions are currently in use or under consideration, with more in development. Since the discovery of stable SiO2-Na2O-CaO ceramic formulations several thousand years ago, most silicate ceramics used by man have an SiO2 content of 65 wt % or more. These SiO2-Na2O-CaO ceramics are more commonly called glass. Although these 65 wt. % silica glasses are extremely bioinert they contain few other desirable qualities. They are weak and shatter easily. The first bioactive glass contained 20-25 wt. % Na2O and 20-25 wt. % CaO and only 45-55 wt. % SiO2. Unfortunately, this new type of glass was still very weak and brittle. The addition of P2O5 to the SiO2-Na2O-CaO matrix makes the glass extremely bioactive.
Bioactive glasses and machinable glass-ceramics are available under a number of trade names. Some of the patented ceramics such as ‘Ceravital’ and ‘Bioglass 45S5’ are so bioactive that within one hour of implantation an HCA layer (precursor to bone [in]growth) nearly 500 nm deep is formed. It has also been claimed that soft tissue growth on the surface of ‘Bioglass 45S5’ has been observed. Alumina and zirconia are among the bioinert ceramics used for prosthetic devices. Porous ceramics such as calcium phosphate-based materials are used for filling bone defects. The ability to control porosity and solubility of some ceramic materials offers the possibility of use as drug delivery systems. Glass microspheres have been employed as delivery systems for radioactive therapeutic agents, for example.
Material selection must also take into consideration the demands of forming complex shapes with strict dimensional tolerances. Devices for use within the body must be able to withstand corrosion in a biological environment and endure use for years without undue wear (and without causing damage to surrounding tissues). Before insertion, they should be unchanged during storage, and must be sterilizable without damage. Materials scientists must keep in mind a multitude of properties and capabilities as they seek to develop the materials that will serve to improve the lives of patients.
In conclusion, the discovery of novel inorganic and polymeric biomaterials, and the refinement of traditional ones, is creating unprecedented excitement in the field as materials designers increasingly confront many of the fundamental challenges of medical science. As the biomaterials discipline itself evolves, the startling advances of the last few years in genomics and proteomics, in various high-throughput cell-processing techniques, in supramolecular and permutational chemistry, and in information technology and bioinformatics promise to support the quest for new materials with powerful analytic tools and insights of boundless energy and sophistication.
Supramolecular Chemistry
Another intriguing new field of great promise is supramolecular chemistry, which is concerned with developing molecular assemblies for biological applications based on macromolecular architectures that mimic nanoscale systems or mechanisms in nature (biomimetics). Novel synthesis methods based on supramolecular chemistry have been used to create branched or graft, cyclic, cross-linked, star, and dendritic polymer structures. A complete list of dendrimers from Sigma-Aldrich is provided in the section on Nanomaterials.
References
To continue reading please sign in or create an account.
Don't Have An Account?