790427C
Avanti
06:0 PC-d22
Avanti Research™ - A Croda Brand 790427C
Synonym(e):
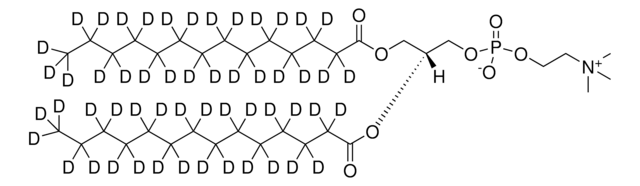
1,2-dihexanoyl-d22-sn-glycero-3-phosphocholine
About This Item
Empfohlene Produkte
Assay
>99% (TLC)
Form
liquid
Verpackung
pkg of 1 × 1 mL (790427C-10mg)
Hersteller/Markenname
Avanti Research™ - A Croda Brand 790427C
Konzentration
10 mg/mL (790427C-10mg)
Versandbedingung
dry ice
Lagertemp.
−20°C
Allgemeine Beschreibung
Anwendung
- as bicelles for lipid in HN-HN nuclear overhauser enhancement (NOE) detection
- as an internal standard in residual algal suspension for liquid chromatography-mass spectrometry analysis (LC-MS/MS)
- to produce bicelles to investigate C-peptide and membrane interaction by diffusion nuclear magnetic resonance (NMR) and 2D total correlation spectroscopy (TOCSY)
Biochem./physiol. Wirkung
Verpackung
Rechtliche Hinweise
Signalwort
Danger
Gefahreneinstufungen
Acute Tox. 3 Inhalation - Acute Tox. 4 Oral - Carc. 2 - Eye Irrit. 2 - Repr. 2 - Skin Irrit. 2 - STOT RE 1 - STOT SE 3
Zielorgane
Central nervous system
WGK
WGK 3
Analysenzertifikate (COA)
Suchen Sie nach Analysenzertifikate (COA), indem Sie die Lot-/Chargennummer des Produkts eingeben. Lot- und Chargennummern sind auf dem Produktetikett hinter den Wörtern ‘Lot’ oder ‘Batch’ (Lot oder Charge) zu finden.
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.