SRP5190
HSP70, His tagged human
recombinant, expressed in baculovirus infected Sf9 cells, ≥70% (SDS-PAGE), buffered aqueous glycerol solution
Synonyme(s) :
HSP70-1, HSP72, HSPA1, HSPA1A, HSPA1B
About This Item
Produits recommandés
Source biologique
human
Produit recombinant
expressed in baculovirus infected Sf9 cells
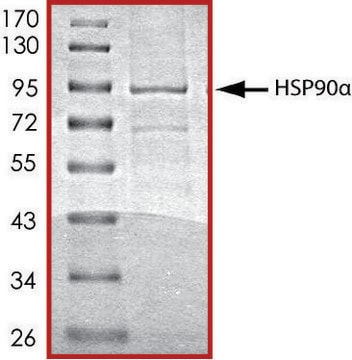
Pureté
≥70% (SDS-PAGE)
Forme
buffered aqueous glycerol solution
Poids mol.
~70 kDa
Numéro d'accès NCBI
Application(s)
cell analysis
Conditions d'expédition
dry ice
Température de stockage
−70°C
Informations sur le gène
human ... HSPA1A(3303)
Description générale
Application
- to study the interaction between HSP70 and receptor of advanced glycation endproducts (RAGE) using protein proximity ligand assay (PLA)
- to facilitate the import of superoxide dismutase 2 (SOD2) into the mitochondria
- to study its role in muscle catabolism
Actions biochimiques/physiologiques
Forme physique
Notes préparatoires
Mention d'avertissement
Danger
Mentions de danger
Conseils de prudence
Classification des risques
Eye Irrit. 2 - Repr. 1B - Skin Irrit. 2
Code de la classe de stockage
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
Classe de danger pour l'eau (WGK)
WGK 1
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique