8.52106
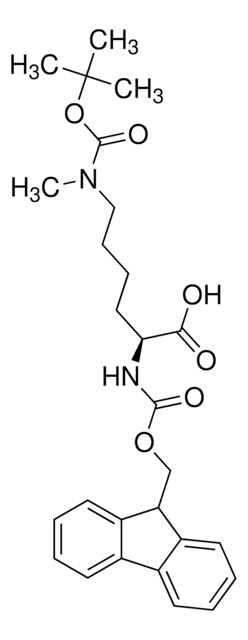
Fmoc-Lys(Me,Boc)-OH
≥98% (TLC), for peptide synthesis, Novabiochem®
Synonyme(s) :
Fmoc-Lys(Me,Boc)-OH, N-α-Fmoc-N-ε-methyl-N-ε-t.-Boc-L-lysine
About This Item
Produits recommandés
product name
Fmoc-Lys(Me,Boc)-OH, Novabiochem®
Niveau de qualité
Gamme de produits
Novabiochem®
Pureté
≥97.0% (HPLC)
≥98% (TLC)
Forme
powder
Capacité de réaction
reaction type: Fmoc solid-phase peptide synthesis
Fabricant/nom de marque
Novabiochem®
Application(s)
peptide synthesis
Groupe fonctionnel
amine
Température de stockage
2-8°C
InChI
1S/C27H34N2O6/c1-27(2,3)35-26(33)29(4)16-10-9-15-23(24(30)31)28-25(32)34-17-22-20-13-7-5-11-18(20)19-12-6-8-14-21(19)22/h5-8,11-14,22-23H,9-10,15-17H2,1-4H3,(H,28,32)(H,30,31)/t23-/m0/s1
Clé InChI
JHMSFOFHTAYQLS-QHCPKHFHSA-N
Description générale
Associated Protocols and Technical Articles
Cleavage and Deprotection Protocols for Fmoc SPPS
Overcoming Aggregation in Fmoc SPPS
Literature references
[1] A.J. Bannister (2002) Cell, 109, 801.
[2] A.E. McBride & P.A. Silver (2001) Cell, 106, 5.
[3] S. Rothbart, et al. (2012) Methods Enzymol., 512, 107.
Liaison
Remarque sur l'analyse
Appearance of substance (visual): powder
Identity (IR): passes test
Optical rotation α 25/D (c=1 in DMF): -13.0 - -8.5 °
Purity (TLC(CMA2)): ≥ 98 %
Assay (HPLC, area%): ≥ 97.0 % (a/a)
Solubility (1 mmole in 2 ml DMF): clearly soluble
To see the solvent systems used for TLC of Novabiochem® products please click here.
Informations légales
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Articles
Unnatural amino acids, the non-proteinogenic amino acids that either occur naturally or are chemically synthesized, are becoming more and more important as tools for modern drug discovery research.
Unnatural amino acids, the non-proteinogenic amino acids that either occur naturally or are chemically synthesized, are becoming more and more important as tools for modern drug discovery research.
Unnatural amino acids, the non-proteinogenic amino acids that either occur naturally or are chemically synthesized, are becoming more and more important as tools for modern drug discovery research.
Unnatural amino acids, the non-proteinogenic amino acids that either occur naturally or are chemically synthesized, are becoming more and more important as tools for modern drug discovery research.
Protocoles
We provide an overview of our available reagents, together with recommendations and details of their use for synthesis of peptides containing post-translationally modified amino acids.
We provide an overview of our available reagents, together with recommendations and details of their use for synthesis of peptides containing post-translationally modified amino acids.
We provide an overview of our available reagents, together with recommendations and details of their use for synthesis of peptides containing post-translationally modified amino acids.
We provide an overview of our available reagents, together with recommendations and details of their use for synthesis of peptides containing post-translationally modified amino acids.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique