SAE0090
Beta-galactoside alpha-2,6-sialyltransferase 1
≥300 units/mg protein, ST6GAL1 human recombinant, expressed in HEK 293 cells
Synonyme(s) :
Alpha 2,6-ST 1, B-cell antigen CD75, CMP-N-acetylneuraminate-beta-galactosamide-alpha-2,6-sialyltransferase 1, ST6Gal I, Sialyltransferase 1
About This Item
Produits recommandés
Produit recombinant
expressed in HEK 293 cells
Description
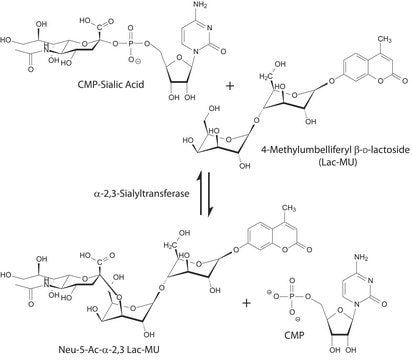
The specific activity of ST6Gal I is measured by its ability to transfer sialic acid from CMP-NANA to asialofetuin.
Pureté
≥95% (SDS-PAGE)
Forme
lyophilized powder
Activité spécifique
≥300 units/mg protein
Conditions d'expédition
ambient
Température de stockage
−20°C
Description générale
Actions biochimiques/physiologiques
Terminal sialylation has been shown to decrease Fcγ receptor binding and increase anti-inflammatory activity,3 as well as antibody-dependent cellular cytotoxicity in different studies by reduced binding of sialylated antibody towards FcγRIIIa.4-5
This recombinant ST6Gal I product can be used to study the mode of action of the enzyme, as well as its potential inhibitors. It can also be used as a glycoengineering tool to modify glycoproteins in vitro.
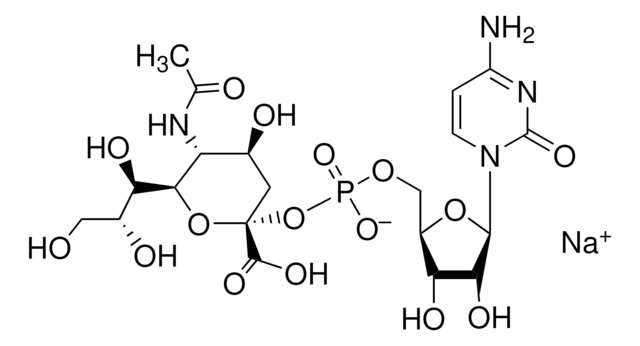
CMP-N-acetylneuraminate (CMP-sialic acid, CMP-NANA) to the β-D-galactosyl-1,4-N-acetyl-D-glucosaminyl termini on glycoproteins.
Sialic acids are distributed in a variety of glycolipids and glycoproteins. The sialic acid that is added to a galactose (Gal) can be bound either to the hydroxyl attached to carbon-3 of Gal to form an α-2,3 glycosidic linkage, or to the hydroxyl group attached to carbon-6 to form an α-2,6 glycosidic linkage. ST6Gal I generates a α-2,6 linkage of sialic acid on the non-reducing, terminal Galβ1 4GlcNAc residues of oligosaccharides and glycoconjugates.
Terminal sialylation has been shown to decrease Fcγ receptor binding and increase anti-inflammatory activity, as well as antibody-dependent cellular cytotoxicity in different studies by reduced binding of sialylated antibody towards FcγRIIIa.
Définition de l'unité
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 2
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Articles
Explore tools for glycosyltransferase synthesis and modification of glycans, such as glycosyltransferases and nucleotide sugar donors.
Explore tools for glycosyltransferase synthesis and modification of glycans, such as glycosyltransferases and nucleotide sugar donors.
Explore tools for glycosyltransferase synthesis and modification of glycans, such as glycosyltransferases and nucleotide sugar donors.
Explore tools for glycosyltransferase synthesis and modification of glycans, such as glycosyltransferases and nucleotide sugar donors.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique