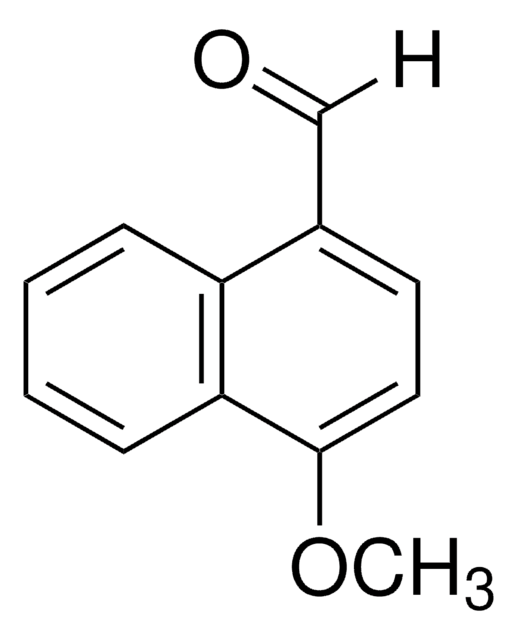
M9894
4-Methoxy-2-naphthylamine
≥98%
Se connecterpour consulter vos tarifs contractuels et ceux de votre entreprise/organisme
About This Item
Formule empirique (notation de Hill):
C11H11NO
Numéro CAS:
Poids moléculaire :
173.21
Numéro MDL:
Code UNSPSC :
12352100
ID de substance PubChem :
Nomenclature NACRES :
NA.22
Produits recommandés
Pureté
≥98%
Forme
solid
Température de stockage
−20°C
Chaîne SMILES
COc1cc(N)cc2ccccc12
InChI
1S/C11H11NO/c1-13-11-7-9(12)6-8-4-2-3-5-10(8)11/h2-7H,12H2,1H3
Clé InChI
SFKZPTYRENGBTJ-UHFFFAOYSA-N
Catégories apparentées
Conditionnement
Bottomless glass bottle. Contents are inside inserted fused cone.
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
Eyeshields, Gloves, type N95 (US)
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
S Scharpé et al.
Clinical chemistry, 34(11), 2299-2301 (1988-11-01)
A new fluorometric assay for determining dipeptidyl peptidase IV (DPP IV; EC 3.4.14.5) was developed. The synthetic substrate glycyl-L-proline-4-methoxy-2-naphthylamide (20 mmol/L), Tris buffer (50 mmol/L, pH 8.3), and serum (20 microL) are mixed and incubated. The reaction is stopped with
S H Randell et al.
The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society, 33(7), 677-686 (1985-07-01)
Dipeptidyl peptidase II (DPP II) in normal rat lung was evaluated by the enzymes' ability to hydrolyze Lys-Ala or Lys-Pro derivatives of 4-methoxy-2-naphthylamine (MNA). For visualization of this activity, the liberated MNA was coupled with fast blue B for light
Epidermal aminopeptidase activity and metabolism as observed in an organized HaCaT cell sheet model.
I Steinsträsser et al.
Journal of pharmaceutical sciences, 86(3), 378-383 (1997-03-01)
Metabolism studies on organized HaCaT keratinocyte cell sheets are reported. Cells were grown on porous membranes to form organized cell sheets of several cell layers, which were considered as a model of viable epidermis. Metabolism was studied by reflection kinetics
Heidi A Kluess et al.
Medicine, 98(13), e14982-e14982 (2019-03-29)
The purpose was to investigate changes in neuropeptide Y (NPY) protein and dipeptidyl peptidase IV (DPP-IV) activity in the plasma and saliva in normally cycling women and women after menopause. We recruited 7 cycling women and 7 postmenopausal women for
C N Kennett et al.
Journal of periodontal research, 29(3), 203-213 (1994-05-01)
Cathepsin B activity was demonstrated histochemically in unfixed cryostat sections of inflamed human gingiva using the 2-methoxy-4-naphthylamide (MNA) substrates Z-Val-Lys-Lys-Arg-MNA and Z-Ala-Arg-Arg-MNA with a post-azo-coupling technique. Enzyme localisation was confirmed by immunocytochemistry with polyclonal sheep anti-human cathepsin B. In both
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique