669032
PEPPSI™-IPr catalyst
98%, Umicore
Synonyme(s) :
[1,3-Bis(2,6-Diisopropylphenyl)imidazol-2-ylidene](3-chloropyridyl)palladium(II) dichloride
About This Item
Produits recommandés
Niveau de qualité
Pureté
98%
Pertinence de la réaction
core: palladium
reagent type: catalyst
Capacité de réaction
reaction type: Buchwald-Hartwig Cross Coupling Reaction
reaction type: Cross Couplings
reaction type: Heck Reaction
reaction type: Hiyama Coupling
reaction type: Negishi Coupling
reaction type: Sonogashira Coupling
reaction type: Stille Coupling
reaction type: Suzuki-Miyaura Coupling
Fabricant/nom de marque
Umicore
Pf
230 °C
Chaîne SMILES
Cl[Pd]Cl.Clc1cccnc1.CC(C)c2cccc(C(C)C)c2N3CN(C=C3)c4c(cccc4C(C)C)C(C)C
InChI
1S/C27H38N2.C5H4ClN.2ClH.Pd/c1-18(2)22-11-9-12-23(19(3)4)26(22)28-15-16-29(17-28)27-24(20(5)6)13-10-14-25(27)21(7)8;6-5-2-1-3-7-4-5;;;/h9-16,18-21H,17H2,1-8H3;1-4H;2*1H;/q;;;;+2/p-2
Clé InChI
BLDKGTGQENJFON-UHFFFAOYSA-L
Description générale
Application
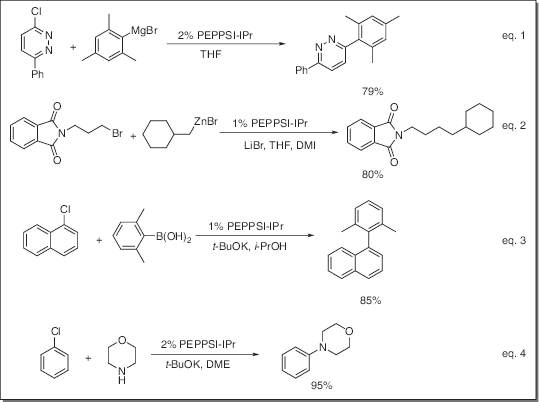
- Catalyst for Kumada-Tamao-Corriu (KTC) reaction (eq. 1)
- Catalyst for Negishi coupling reaction (eq. 2)
- Catalyst for Suzuki coupling reaction (eq. 3)
- Catalyst for Buchwald-Hartwig amination reaction (eq. 4)
For small scale and high throughput uses, product is also available as ChemBeads (931063)
Informations légales
This product, its manufacturing or use, is the subject of one or more issued or pending U.S. Patents (and foreign equivalents) owned or controlled by Umicore PMC. The purchase of this product from Umicore PMC through Sigma-Aldrich, its affiliates or their authorized distributors conveys to the buyer a limited, one-time, non-exclusive, non-transferable, non-assignable license. Buyer′s use of this product may infringe patents owned or controlled by third parties. It is the sole responsibility of buyer to ensure that its use of the product does not infringe the patent rights of third parties or exceed the scope of the license granted herein.
For any further information on product please refer to your local Umicore PMC contact at http://www.pmc.umicore.com
Patented, U.S. Pat. No. 7,250,510. Sold under an exclusive license from Total Synthesis Ltd.
Mention d'avertissement
Warning
Mentions de danger
Conseils de prudence
Classification des risques
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
dust mask type N95 (US), Eyeshields, Gloves
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Articles
Professor Mike Organ and co-workers have developed the PEPPSI™ (Pyridine-Enhanced Precatalyst Preparation Stabilization and Initiation) precatalysts for palladium-catalyzed cross-coupling reactions.
PEPPSI™ palladium N-heterocyclic-carbene catalyst system enhances efficiency and functional group tolerance in catalysis.
PEPPSI™ palladium N-heterocyclic-carbene catalyst system enhances efficiency and functional group tolerance in catalysis.
PEPPSI™ palladium N-heterocyclic-carbene catalyst system enhances efficiency and functional group tolerance in catalysis.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique





![[Pd(IPr#)(cin)Cl]](/deepweb/assets/sigmaaldrich/product/structures/391/578/9bb7eaef-fa70-4f50-8644-2c55cec3925d/640/9bb7eaef-fa70-4f50-8644-2c55cec3925d.png)
![[1,1′-bis(diphénylphosphino)ferrocène]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)




