238198
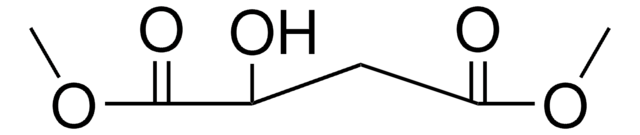
Dimethyl maleate
96%
Synonyme(s) :
(2Z)-2-Butenedioic acid dimethyl ester, (Z)-2-Butenedioic acid dimethyl ester, (Z)-Dimethyl 2-butenedioate, Dimethyl (Z)-but-2-enedioate
About This Item
Produits recommandés
Pureté
96%
Forme
liquid
Impuretés
≤4% dimethyl fumarate
Indice de réfraction
n20/D 1.441 (lit.)
Point d'ébullition
204-205 °C (lit.)
Solubilité
water: soluble 77.9 g/L at 20 °C
Densité
1.152 g/mL at 25 °C (lit.)
Chaîne SMILES
[H]\C(=C(/[H])C(=O)OC)C(=O)OC
InChI
1S/C6H8O4/c1-9-5(7)3-4-6(8)10-2/h3-4H,1-2H3/b4-3-
Clé InChI
LDCRTTXIJACKKU-ARJAWSKDSA-N
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Description générale
Dimethyl maleate (DMM) is a reactive dienophile and undergoes ultrasonic irradiation promoted Diels-Alder reaction with substituted furans. Mesoporous siliceous SBA-15-supported Cu catalyzed gas phase hydrogenolysis of DMM to 1,4-butanediol (BDO) has been reported. Aluminium chloride has been reported to accelerate the Diels-Alder reaction of DMM and anthracene. DMM can be synthesized by the esterification of maleic anhydride with sulfuric acid and methanol.
Application
- Dissociation of bovine 6S procarboxypeptidase A by reversible condensation with 2,3-dimethyl maleic anhydride: application to the partial characterization of subunit III.: This study explores the dissociation of bovine procarboxypeptidase A using 2,3-dimethyl maleic anhydride, highlighting its applications in the partial characterization of enzyme subunits. The research demonstrates the potential of dimethyl maleate derivatives in protein chemistry and enzyme structure studies. (Puigserver and Desnuelle, 1975).
Mention d'avertissement
Danger
Mentions de danger
Conseils de prudence
Classification des risques
Acute Tox. 3 Dermal - Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Sens. 1 - STOT RE 2 Dermal - STOT SE 3
Organes cibles
Respiratory system, Skin
Code de la classe de stockage
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
Classe de danger pour l'eau (WGK)
WGK 1
Point d'éclair (°F)
203.0 °F - closed cup
Point d'éclair (°C)
95 °C - closed cup
Équipement de protection individuelle
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Articles
The Diels–Alder reaction is the reaction between a conjugated diene and an alkene (dienophile) to form unsaturated six-membered rings. It is also referred to as a cycloaddition.
The Diels–Alder reaction is the reaction between a conjugated diene and an alkene (dienophile) to form unsaturated six-membered rings. It is also referred to as a cycloaddition.
The Diels–Alder reaction is the reaction between a conjugated diene and an alkene (dienophile) to form unsaturated six-membered rings. It is also referred to as a cycloaddition.
The Diels–Alder reaction is the reaction between a conjugated diene and an alkene (dienophile) to form unsaturated six-membered rings. It is also referred to as a cycloaddition.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique