220868
1-Acetoxy-1,3-butadiene
mixture of cis and trans
Synonyme(s) :
1,3-Butadienyl acetate
About This Item
Produits recommandés
Pression de vapeur
40 mmHg ( 60 °C)
Forme
liquid
Contient
0.1% p-tert-butylcatechol as stabilizer
Indice de réfraction
n20/D 1.469 (lit.)
Point d'ébullition
60-61 °C/40 mmHg (lit.)
Densité
0.945 g/mL at 25 °C (lit.)
Température de stockage
2-8°C
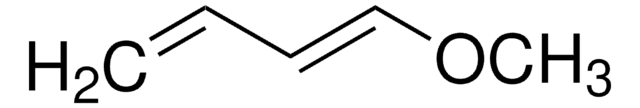
Chaîne SMILES
CC(=O)O\C=C\C=C
InChI
1S/C6H8O2/c1-3-4-5-8-6(2)7/h3-5H,1H2,2H3/b5-4+
Clé InChI
NMQQBXHZBNUXGJ-SNAWJCMRSA-N
Description générale
Application
- Diels-Alder reaction with ortho-carbazolequinones to yield benzocarbazolequinone.
- Diels-Alder reaction with diethyl ketovinylphosphonate, with and without Lewis acid assistance.
- Diels-Alder reaction with methyl acrylate to yield racemic forms of 2-hydroxy-3-cyclohexenecarboxylic acid.
It was used for the reaction with dienophiles such as maleimides, a juglone, a butyne-1,4-dione and methyl 2-(4-methylphenyl)-2H-azirine-3-carboxylate and during visible light photocatalysis. It was also used as reactant during intermolecular oxa-Pictet-Spengler cyclization.
Mention d'avertissement
Danger
Mentions de danger
Conseils de prudence
Classification des risques
Acute Tox. 3 Dermal - Acute Tox. 4 Oral - Eye Irrit. 2 - Flam. Liq. 3 - Skin Irrit. 2 - STOT SE 3
Organes cibles
Respiratory system
Code de la classe de stockage
3 - Flammable liquids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
91.4 °F - closed cup
Point d'éclair (°C)
33 °C - closed cup
Équipement de protection individuelle
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Articles
The Diels–Alder reaction is the reaction between a conjugated diene and an alkene (dienophile) to form unsaturated six-membered rings. It is also referred to as a cycloaddition.
The Diels–Alder reaction is the reaction between a conjugated diene and an alkene (dienophile) to form unsaturated six-membered rings. It is also referred to as a cycloaddition.
The Diels–Alder reaction is the reaction between a conjugated diene and an alkene (dienophile) to form unsaturated six-membered rings. It is also referred to as a cycloaddition.
The Diels–Alder reaction is the reaction between a conjugated diene and an alkene (dienophile) to form unsaturated six-membered rings. It is also referred to as a cycloaddition.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique