215376
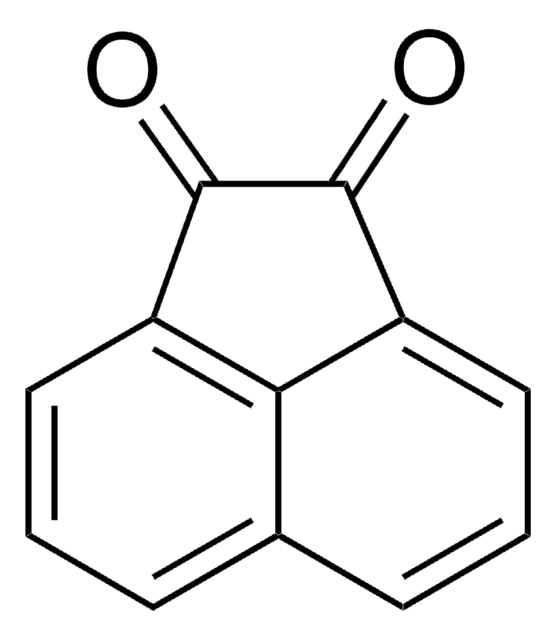
Acénaphtène
99%
Synonyme(s) :
1,8-éthylènenaphthalène
About This Item
Produits recommandés
Densité de vapeur
5.32 (vs air)
Niveau de qualité
Pression de vapeur
10 mmHg ( 131 °C)
Pureté
99%
Forme
solid
Point d'ébullition
279 °C (lit.)
Pf
90-94 °C (lit.)
Solubilité
chloroform: soluble 5%, clear, colorless to faintly yellow
Chaîne SMILES
C1Cc2cccc3cccc1c23
InChI
1S/C12H10/c1-3-9-4-2-6-11-8-7-10(5-1)12(9)11/h1-6H,7-8H2
Clé InChI
CWRYPZZKDGJXCA-UHFFFAOYSA-N
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Catégories apparentées
Description générale
Bacterial oxidation of acenaphthene by Beijerinckia sp. and Beijerinckia sp. strain B8/36 has been reported. Acenapthene forms 1:1:1 inclusion compound by complexing with inclusion compound of β-cyclodextrin and alcohol. Kinetics of the atmospherically important gas-phase reactions of acenaphthene with OH and NO3 radicals, O3 and N2O5 has been investigated at 296 ± 2K.
Application
- Optimization of Acenaphthene Production: A research article demonstrated the use of high-efficiency HILIC capillary columns for slurry packing at 2100 bar, showcasing an advanced technique that could optimize the synthesis and purification of complex polycyclic aromatic hydrocarbons like acenaphthene, essential for enhancing productivity and purity in chemical processes (Anderson et al., 2024).
- Environmental Impact Assessment: A study investigated the relationship between polycyclic aromatic hydrocarbons (PAHs) and reactive oxygen species in PM2.5 emissions, providing a critical evaluation of the environmental impacts of compounds like acenaphthene in industrial regions, thus informing pollution control and environmental safety strategies (Xu et al., 2024).
- Advances in Organic Semiconductor Materials: Research focused on the synthesis and properties of pyrene-bridged acenaphthenes, contributing to the development of novel organic semiconductor materials, which are crucial for electronic and photonic applications, thus broadening the utility of acenaphthene in high-tech industries (Polkaehn et al., 2023).
- Bioassays for Organic Pollutants: Researchers developed bioassays for organic pollutants, including acenaphthene, using chemical activity-based loading of artificial sediments. This approach offers a new method to assess ecological risks associated with organic contaminants, while also optimizing the derivation of predicted no-effect concentrations (PNECs) for polycyclic aromatic hydrocarbons, critical for regulatory compliance and environmental health. (Abel et al., 2024), (Sun et al., 2023).
Mention d'avertissement
Warning
Mentions de danger
Conseils de prudence
Classification des risques
Aquatic Acute 1 - Aquatic Chronic 1
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
257.0 °F - closed cup
Point d'éclair (°C)
125.0 °C - closed cup
Équipement de protection individuelle
dust mask type N95 (US), Eyeshields, Gloves
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique