137448
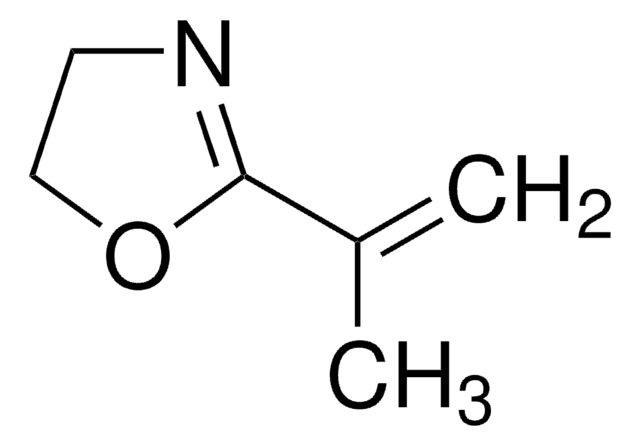
2-Methyl-2-oxazoline
98%
Synonym(s):
2-Methyloxazoline
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C4H7NO
CAS Number:
Molecular Weight:
85.10
Beilstein:
104227
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
liquid
refractive index
n20/D 1.434 (lit.)
bp
109.5-110.5 °C (lit.)
density
1.005 g/mL at 25 °C (lit.)
SMILES string
CC1=NCCO1
InChI
1S/C4H7NO/c1-4-5-2-3-6-4/h2-3H2,1H3
InChI key
GUXJXWKCUUWCLX-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
2-Methyl-2-oxazoline undergoes polymerization with 2-butyl-2-oxazoline to form poly(2-oxazoline) block copolymer. It undergoes cationic ring-opening polymerization with 2-(dec-9-enyl)-2-oxazoline to yield copoly(2-oxazoline)s.
Application
2-Methyl-2-oxazoline was used in cationic polymerization of a series of linear 2-alkyl-2-oxazolines under microwave irradiation.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Flam. Liq. 2
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
68.0 °F - closed cup
Flash Point(C)
20 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Yalin Zhang et al.
Talanta, 150, 375-387 (2016-02-04)
Nitrogen-rich melamine (66% by mass) had been found to be illegally adulterated to milk products and animal feed in order to increase the apparent protein content, and ingestion of melamine may lead to the formation of kidney stones. Hence, the
Laetitia Korchia et al.
Soft matter, 13(25), 4507-4519 (2017-06-07)
A series of amphiphilic photo-responsive heterografted copolymers have been successfully synthesized. The random copolymers were composed of a methacrylate backbone, with various compositions of hydrophilic oligomeric 2-methyl-2-oxazoline side chains (OMOx) and hydrophobic long alkyl chains terminated by a coumarin moiety
Ye Han Yan et al.
Journal of biomaterials science. Polymer edition, 31(4), 423-438 (2019-12-04)
In this work, the comb-like poly(2-methyl-2-oxazoline) copolymer, poly(2-aminoethyl methacrylate-random-poly(2-methyl-2-oxazoline) (PMOXA-r-AEMA, PMA) is synthesized, and the CuSO4/H2O2-triggered dopamine/PMA co-deposition process is investigated. Ellipsometry, water contact angle (WCA), and X-ray photoelectron spectroscopy (XPS) are used to characterize the thickness, hydrophilicity, and surface
Ondrej Sedlacek et al.
Journal of controlled release : official journal of the Controlled Release Society, 326, 53-62 (2020-06-23)
Poly(2-oxazoline)s represent an emerging class of polymers with increasing potential in biomedical sciences. To date, most of the work on poly(2-oxazoline)-drug conjugates focused on poly(2-ethyl-2-oxazoline) (PEtOx), a biocompatible water-soluble polymer with biological properties similar to polyethylene glycol. However, the more
Denial Mahata et al.
Scientific reports, 7, 46412-46412 (2017-04-13)
Lignin, one of the most abundant renewable feedstock, is used to develop a biocompatible hydrogel as anti-infective ointment. A hydrophilic polyoxazoline chain is grafted through ring opening polymerization, possess homogeneous spherical nanoparticles of 10-15 nm. The copolymer was covalently modified with
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service