Understanding Sweet Taste Perception
The powerful effect of the pleasurable sensation arising from sweet tasting foods has led to consumer demand and large-scale production of the caloric sweetening agents sucrose and fructose. Because of economic considerations and sugar shortages, consumer demand for alternative sweeteners emerged during World War II. During this time period a small consulting company in Saint Louis, Missouri, USA developed a saccharin production process to meet the demand for alternative sweeteners. The success of saccharin as a breakthrough product for this company launched the Sigma-Aldrich® Corporation. This need for alternative sweeteners continues to grow as commercial food suppliers, such as Nestlé, form sensory research collaborations to develop customized foods that meet specific customer taste preferences while providing optimized nutritional benefits. This momentum combined with the highly publicized, albeit inconclusive, relationship between high-fructose corn syrup and pre-disease conditions, specifically insulin resistance, are driving the search to elucidate the genetic blueprint, protein configurations and signaling mechanisms underlying sweet taste perception.
The expansion of novel natural, synthetic, and semi-synthetic sweetener alternatives with negligible caloric contribution and ideal taste will continue as new findings in sweet taste perception are revealed. The first generation of sweetener alternatives includes the synthetic sweeteners saccharin, cyclamate, and aspartame. These compounds have been studied in animal models for their carcinogenic potential but the newer generation of synthetic sweeteners, acesulfame-K, neohisperidin, alitame, and sucralose have not been suspected to cause cancer nor are they genotoxic.
Mammalian Sweet Taste Perception
The human taste modality for sweetness was originally thought to correlate to a distinct, regional lingual tissue location. Genomic screening and knockout methodology have since supported the counter argument that sweet taste sensation in humans is derived from the varied expression of G protein-coupled receptors (GPCRs) throughout lingual, palatal and epiglottis tissue surfaces.
In humans, the olfactory response proceeds directly from an odorant to a neural response but the gustatory response proceeds from an interaction between a tastant and a taste receptor and then to a neural response. Taste receptor cells are clustered in taste buds distributed along palatal and lingual locations throughout the human oral cavity.1 The receptor proteins within these taste cells bind sweet ligands, eliciting a downstream signaling cascade through activated synapses to excited sensory nerve fibers that carry the signal to the brain for central taste processing.2 Yi-Jen Huang et al. implicated serotonin as a responsible synaptic transmitter by monitoring serotonin release with biosensor CHO cells after sweet stimulation3 and Heath et al. describe serotonin as a modulator for the human sweetness threshold based on human taste function testing.4
The saccharin preference (Sac) locus in the mouse genome is a site associated with sweetness variability for natural sugars and synthetic sweeteners. Several research groups successfully searched near the Sac locus to uncover genes responsible for human sweetness taste perception. For example, Bachmanov et al. used a positional cloning approach to identify the gene Tas1r3 that encodes the G proteincoupled receptor, T1R3.5 A different study confirmed that the Sac locus encodes mammalian T1R3, but also showed that a second receptor, T1R2, combines with T1R3 to detect sweetness.6 The structure formed between T1R2 and T1R3 is a heterodimer with at least four sweet tastant binding sites, including a special binding location that is accommodating to sweet proteins. The binding sites were confirmed by calculating the free energy of various molecular tastants.7
Crystallography has been used to determine the binding properties of sweet proteins to this special binding location.8-10 The sweetness property of these proteins is inhibited in a riboflavin independent manner by riboflavin-binding protein (RBP), unlike sucrose and other low-molecular weight sweeteners including saccharin and stevioside. RBP inhibition is a result of RBP binding to the sweet taste receptors rather than RBP-mediated sweet protein degradation.11 It is suggested that RBP inhibits an unique sweet protein-binding site distinguishable from the binding sites specific for low-molecular weight sweeteners. A wedge model for this special binding site has been proposed with data from an electrostatic study of monellin mutants.12 Monellin is one of several sweet proteins described in Table 1.13
Synergy between certain sweeteners is suspected to be a result of multiple but unique sweeteners binding on the same receptor but at different sites based on optimal sweetener conformation and molecular size.14 Amino acid domain variation between species results in different taste receptor binding properties. For example, the flavonoid sweetening agent neohesperidin dihydrochalcone binds to the heptohelical domain of human T1R3 but not rat T1R3.15
The T1R3 and T1R2 receptors are each comprised of seven transmembrane α-helical segments. These receptors are expressed not just in the oral cavity of various species but also in the intestinal tract.16 When an extracellular sweet tastant binds to the T1R2/T1R3 heterodimer, a conformational change occurs that allows the intracellular G protein to interact with the receptor.17 The coupled G protein has not been clearly identified; however, both bitter and sweet tasting cells have been linked to the G protein, α-gustducin. The varied co-expression of α-gustducin and the T1R receptors indicate that α-gustducin may be the responsible G protein for sweetness taste perception under some but not all circumstances. Different taste cell locations have distinct α-gustducin and receptor co-expression patterns; lingual taste cells have less co-expression than palatal taste cells. In a particular murine study, T1R2-positive palatal cells had a 38% gustducin co-expression rate and T1R3-positive palatal taste cells had a 100% gustducin co-expression rate.18
Upon sweet tastant binding to the GPCR, the G protein initiates a downstream transduction cascade (Figure 1). The G protein modifies the activity of adenylyl cyclase (AC) resulting in the synthesis of the cyclic nucleotide, cAMP.19 If the sweet tastant is a carbohydrate, the cAMP second messenger alters intracellular calcium ion concentrations with calcium uptake from the extracellular space. In contrast, if the sweet tastant is a synthetic compound, calcium is released from intracellular stores through the phospholipase C-mediated conversion of phosphatidylinositol lipids to diacylglycerol and inositol triphosphate (IP3).6,20 The intermediate steps in the synthetic sweetener triggered cascade are not fully understood, however activation of protein kinase C (PKC) is suggested as indicated by inhibition studies with the PKC inhibitor, bisindolylmaleimide (BIM).21
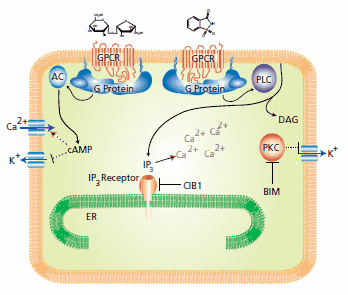
Figure 1.The mechanism for the carbohydrate sucrose (left) differs from the mechanism for the synthetic sweetener saccharin (right). See text for specific explanation.
These signaling cascades lead to an efflux of potassium ions, potassium conductance inhibition, and membrane depolarization. The increased intracellular calcium concentration is needed for synaptic activation and nerve fiber excitation. RNA interference was used to locate a T1R2/T1R3 interacting protein, named calcium and integrin-binding protein 1 (CIB1), which functions as an in vivo inhibitor of the IP3-dependent calcium release in taste signal transduction. The inhibitory effect of CIB1 was confirmed using a FURA-2 intracellular calcium detection method.22
Current Sweetener Alternatives
Alternative natural sweeteners such as sweet proteins, plant extracts and sugar alcohols, are being further investigated for their optimal physical properties (e.g., melting point) and beneficial physiological impact to determine if they will be suitable as long-term sugar substitutes. Stevioside, the diterpenoid glycosidic constituent of the Stevia rebaudiana Bertoni plant (Cat. No. S5381), has recently gained media attention as Cargill™, in partnership with Coca-Cola®, introduced a powdered stevioside formulation, termed Truvia™, to consumers in the United States. However, stevioside is not being used as a food additive in the United States; it has not been granted a generally recognized as safe (GRAS) status by the U.S. Food and Drug Administration. The status may be revised as stevioside and rebaudioside A, a second glycosidic constituent of Stevia rebaudiana, have been reported to have very low to negligible toxicity levels.23,24
The increased interest in stevioside not only stems from its natural sweetening property but also for its antioxidant, immunomodulatory,25 and anticancer activities. It is also a prospective drug candidate for type II diabetes mellitus. In vitro studies monitoring stevioside and rebaudioside A treatment indicate an enhancement of glucose-dependent insulin secretion. This is a beneficial outcome that counteracts depleted insulin secretion from the extended use of sulfonylurea therapy for diabetes. Depleted insulin secretion is linked to hypoglycemia and secondary drug failure.26,27 The stevioside metabolite, steviol, displays immunomodulatory and anticancer activities. Specifically, steviol was shown to suppress TNF-α induced IL-8 release on human colon carcinoma cell lines. Since steviol is formed in the cecum or colon, its use in balancing coloncyte inflammatory and secretory response will evoke continued research interest.28
As previously mentioned the sweet proteins possess potential for use in sucrose and fructose replacement. The sweet proteins are natural proteins with unique sequence composition and structural variation that impart an intensely, sweet sensation to humans, apes, and old world monkeys but not to all mammalian species.29 The sweet proteins are an ideal sweetener alternative due to both their non-toxic status, based on animal model evidence, and absence of downstream insulin response.30 The sweet proteins and their defining properties are referenced in Table 1. Methods for sweet protein production using bacteria, yeast, fungi, transgenic plant systems, and solid-phase synthesis have been reported.31
Additionally, there are several L-amino acids that elicit sweet perception in humans. Using a taste aversion assay method, it was determined that mice were able to discriminate sucrose from both L-serine and L-threonine. Further research is needed to determine if the sweet-tasting L-amino acids bind to the T1R2/T1R3 heterodimer receptor.32
The unveiling of the ligands, receptor, and transduction participants involved in sweetness perception over the past decade represents a significant contribution to human chemoreception research. This type of research adds to the overall understanding of human decision-making based on chemical, emotional, and physical sensation-based input. The intersection of chemoreception research and business enterprise will result in the availability of novel food alternatives and potential to halt the growing number of diabetes and obesity patients.
References
Pour continuer à lire, veuillez vous connecter à votre compte ou en créer un.
Vous n'avez pas de compte ?