Duolink® PLA Troubleshooting Guide
Tips & Tricks
The Duolink® PLA technology for protein detection offers a simple and straightforward protocol that has a set of defined steps. This section will delineate important reminders that will ensure your Duolink® PLA experiment proceeds smoothly.
Upfront Considerations
- Use conditions for optimal primary antibody performance within the sample to be tested. These include sample processing parameters (fixation, permeabilization, antigen retrieval, etc), primary antibody titer, and incubation time.
- Always include both technical and, when possible, biological controls in your experiment to properly evaluate the results.
- The Duolink® PLA Control Kit is a convenient tool to test the technology and obtain successful results. It includes microscope slides with pre-plated cells and a pair of antibodies to look at protein-protein interaction using the Duolink® PLA reagents. The PLA signals are guaranteed. Use this Control Kit to gain confidence with the Duolink® PLA technology or to add additional controls to your experiment.
Common Experimental Parameters
- Never let your sample dry out. Use a humidity chamber during incubation steps.
- Make sure to remove excess wash solutions from samples. Residual wash buffer can cause further dilution of antibodies and/or decrease ligation or amplification efficiency.
- Use a hydrophobic pen to surround the sample. When using chambered slides, this will help prevent cross-contamination of solutions between wells. When using tissue sections, this will minimize required reagent volume.
- Perform all steps at the appropriate temperatures and incubation times for best results, in particular the enzymatic steps (ligation and amplification).
- For detection of low-abundance proteins, extended amplification times may be required. If background increases under these conditions, perform the amplification and detection steps separately by using the Duolink® Brightfield Amplification Buffer (does not contain detection oligos), followed by a 30-minute incubation with the Duolink® Fluorescent Amplification Buffer (contains detection oligos).
- Perform washes in ample wash buffer and ensure the samples are fully covered. Use Wash Buffers A and B where specified. Perform washes at room temperature.
- Keep enzymes in a freezer block while in use. Make sure other reagents are completely thawed (e.g., ligation buffer and amplification buffer) and vortexed prior to usage.
Sample Storage and Analysis
- All Duolink® PLA Probes must be stored at 4 °C; this includes those generated using Duolink® Probemaker. Do not freeze or results will significantly decrease.
- For fluorescent applications, store slides in the dark at 4 °C after mounting with Duolink® Mounting Media with DAPI for up to 4 days. Seal and store at -20 °C for up to 6 months.
- Do not overexpose. Fluorescent signals can coalesce making it difficult to obtain accurate quantitative analysis. Of note, coalescence will not affect flow cytometry analysis.
- Images should be taken with the same acquisition parameters between experimental and control samples.
Troubleshooting
Despite all precautions, and the use of some excellent tips and trick, sometimes the results are not as expected. Lack of signal in a positive control or high background are two of the most common problems that are often seen when performing immunodetection experiments, and may also occur with the Duolink® PLA technology. The tables below list the probable causes and suggested solutions to get around these common problems. If problems remain, contact our Technical Service online or at (800) 325-5832.
A. Too High of Background Signal
| Concentration of primary antibody is too high
|
|
| Unspecific binding of primary antibodies
|
|
| Insufficient blocking
|
|
| Insufficient washing of reaction |
High level of nonspecific signal (away from cell) due to insufficient washing. |
| Drying of sample |
|
| Precipitate in ligation buffer |
|
| Dust, salt, or fixation precipitates cause highly fluorescent particles |
|
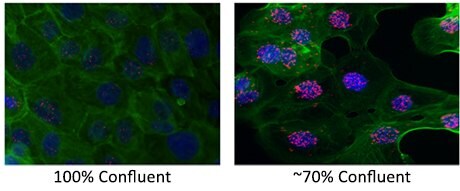
| Cells at suboptimal density |
|
| No or insufficient binding of primary antibodies |
|
| Inappropriate incubation temperatures |
|
| Insufficient removal of excess wash buffers |
|
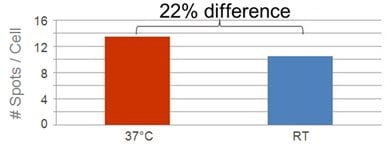
| Wash buffers used incorrectly |
Effects of Wash Buffer Temperature 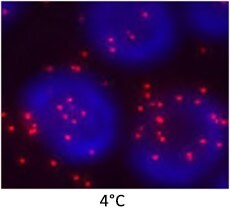 |
| Wrong filter used for acquisition |
|
| Inefficient ligation |
|
| Inefficient amplification |
|
| Coalescence of Signal |
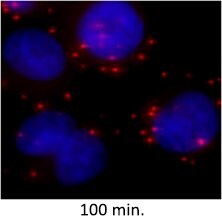 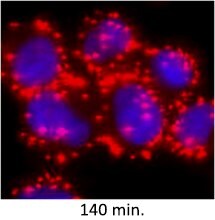
|
| Autofluorescence |
|
Frequently Asked Questions
This section provides answers to some frequently asked questions (FAQs). However, some information is proprietary and cannot be disclosed. This includes questions pertaining to specific composition of reagents and buffers, oligonucleotide length and sequences, or the conjugation chemistries of the Duolink® PLA Probes and Duolink® Probemaker kits.
GENERAL QUESTIONS
- What are the minimum and maximum distances for proximity ligation to work?
All Duolink® PLA products are designed to detect proximity. When using the secondary antibody approach, the theoretical maximum distance between your two target proteins (epitopes) is 40 nm to be able to create a signal. These numbers are based on the average diameter of an antibody being around 10 nm. Duolink® PLA can in theory detect epitopes that are within zero nm of each other, as long as the two primary antibodies can bind and there is no steric hindrance.

- How long do the Duolink® PLA products/reagents last?
- All the reagents in the detection kits must be kept at -20 °C at all times. This is especially critical for the Ligase and the Polymerase enzymes, which need to be kept in a pre-chilled ice-block if taken out from the freezer (an ice-block keeps the enzymes at -20 °C; you cannot achieve that by just having the enzymes on an ice bath). Duolink® PLA probes, antibody diluents, blocking solutions and mounting medium must be kept at +4 °C. Do not freeze the PLA probes or those generated by Probemaker.
- We have a 6-month guarantee upon arrival to your laboratory for the Duolink® PLA Probemaker, as long as it is kept at -20 °C. Once the conjugation has taken place, we have noticed that for most conjugated antibodies the shelf life at +4 °C is three to six months. In this case it will be very much dependent on if the antibody requires special preservatives or not.
- Can I apply Duolink® PLA to live cells or non-fixed tissue samples?
- Duolink® PLA has been optimized for fixed cells and tissues.
- Permeabilization is necessary for the antibodies and probes to find their targets.
- Furthermore, some of the Duolink® PLA reagents (enzymes, DNA oligos) will probably be destroyed by active mechanisms in living cells.
- Can I combine a PLA probe and traditional immunofluorescence?
Yes. It is possible to combine Duolink® PLA and traditional immunofluorescence techniques, as long as all primary antibodies are from different species and the fluorescence detection systems use different colored fluors.
Duolink® PLA Protocol or Analysis
- What are the Duolink® PLA Probes?
- Duolink® PLA Probes are secondary antibodies conjugated with either the PLUS or the MINUS oligonucleotides.
- The secondary antibodies are derived from farmed donkey (Equus asinus domesticus).
- Made using anti-mouse, anti-rabbit, and anti-goat polyclonal antibodies.
- Minimal cross-reactivity with other species.
- What is the minimum and maximum number of cells / tissue needed?
- In principle, the minimum number of cells required for a Duolink® PLA experiment is one cell. The actual minimum number of cells used will be dependent on your particular application.
- The maximum is difficult to establish. Antibodies and reagents may have difficulty to reach cells in the center of the sample if the cell density is too high. 50-70% confluent is optimal.
- When it comes to tissue samples, there is no minimum number. As for the maximum, up to 30 µM thick tissue slices have been used with successful results. 5-10 µM thick is optimal.
- The success of the experiment will depend both on the tissue sections and its pretreatment (e.g. fixation, permeabilization, epitope retrieval).
- When can I stop the procedure? OR What is a good stopping point in the protocol?
- There are several stopping points in the Duolink® PLA protocol. These include:
- After fixation, samples can be left in 1x PBS at 4 °C for several days
- Blocking and primary antibody incubations can be run overnight. Depending on the primary antibody, incubation times can vary from half an hour to overnight. Observe that even if the recommended incubation time is shorter for a particular primary antibody, it is possible to prolong the incubation time to overnight at 4 °C.
- After the procedure is finished.
- For the fluorescence detection: the drying of the slides at room temperature after the final wash step and before mounting the slides can be done overnight. Mounted slides can be stored at +4 °C up to four days or sealed and stored at -20 °C for months before analysis. Please note that slides should be stored in the dark!
- For the HRP detection (Brightfield): after the last dehydration step, slides can be left in fresh xylene or one can proceed with the mounting of the slides. Once slides are mounted they need to get dry, usually overnight.
- Note: The ligation and amplification conditions have been optimized and should not be varied. Detection of a very low abundance protein or flow cytometry application may be an exception but may result in increased background if the amplification and detection steps are not separated.
- There are several stopping points in the Duolink® PLA protocol. These include:
- What is the effect of changing incubation times and temperatures?
The Duolink® PLA protocol has been optimized to obtain results in the shortest time possible without compromising the quality of results. Optimization of incubation times and temperatures has been an important factor in that respect, and therefore, best results are acquired when the protocol is followed with the recommendations given therein.
- How do I perform a single recognition experiment?
Performing a single recognition experiment, you will need one primary antibody against your particular target protein and two PLA probes, both directed towards the species of your primary antibody (e.g. if your primary antibody was raised in rabbit, then you will need the PLA probes anti-Rabbit PLUS and anti-Rabbit MINUS). The rest of the Duolink® PLA experiment is performed as usual.
- How long does the PLA signal last? OR Is the PLA signal stable? OR How should I save my glass slide in order to keep the PLA signals?
- We highly recommend using Duolink® PLA Mounting Media with DAPI for fluorescence applications, because they help to preserve PLA signals. PLA signals can fade when other mounting media is applied, sometimes even immediately after their application on the sample.
- Slides mounted with Duolink® PLA Mounting Medium with DAPI can be stored at 4 °C in darkness up to 4 days before they are analyzed with the microscope.
- Unmounted slides can also be saved at -20 °C over the weekend.
- For long term storage, we recommend applying transparent nail polish around the borders of the cover slip. In this way, the slide can be stored at -20 °C for up to half a year, although normally the signal will fade a little bit with time. If the slide has already been analyzed with the microscope, additional fading may be expected.
- When slides are mounted with an non-aqueous (xylene based), permanent (hard-set) mounting media for the brightfield application, samples can be stored at room temperature and PLA signals will last for years.
Specific Requirements
- What are the Primary Antibody requirements when using Duolink® PLA Probes? OR Which antibodies are recommended?
- The primary antibodies should be:
- IgG-class
- Specific for the target to be detected, preferably affinity purified
- Host must be mouse, rabbit or goat
- Monoclonal or polyclonal
- Validated by IF and/or IHC; PLA validated antibodies are now available.
- We offer over 70,000 primary antibodies for you to consider.
- We have partnered with Bethyl Laboratories to provide validated primary antibody pairs for PLA.
- The primary antibodies should be:
- What concentration of my primary antibody should I use?
If you already have a working assay for IHC or IF, use the same primary antibody concentration to start with. Sometimes it may be necessary to perform a titration of your primary antibody.
Duolink® Probemaker
- My antibodies are from a species other than mouse, rabbit, and goat. Can I still apply Duolink® PLA?
Yes. We offer the Duolink® PLA Probemaker kit that enables conjugation of the PLA oligonucleotide arms PLUS or MINUS to your antibodies of interest. In that way, you build your own PLA probes that can later be applied to detect your proteins of interest in combination with Duolink® PLA Reagents. - Both my primary antibodies are from the same species. Can I still apply Duolink® PLA?
Yes. We offer the Duolink® PLA Probemaker kit that enables conjugation of the PLA oligonucleotide arms PLUS or MINUS to your primary antibodies of interest. In that way, you build your own PLA probes that can later be applied to detect your proteins of interest in combination with Duolink® PLA Reagents. This allows for the detection of protein homodimers. - When should the Probemaker Kit be used?
The Probemaker kit enables conjugation of a PLA oligo (PLUS or MINUS) to any antibody. Thus, PLA probes can be generated using Probemaker from primary antibodies for direct detection or from secondary antibodies for indirect detection.- When the host of a primary antibody is different than mouse, rabbit or goat
- When using two primary antibodies from the same species
- When using a primary antibody from the same host species as the sample (e.g., “mouse on mouse”)
- Are there antibody recommendations for the Probemaker Kit?
- Antibody concentration of 1 mg/mL
- Amount of antibody is 20 mg per conjugation
- Antibody needs to be free of additives
- When using Duolink® PLA Probemaker, can I use a lower concentration of the primary antibody than 1 mg/mL?
- Each kit of Duolink® PLA Probemaker contains reagents to conjugate 20 ug of antibody at a concentration of 1 mg/mL. The use of antibodies at this concentration is strongly recommended.
- Good results cannot be guaranteed with a lower or higher concentration.
- If the antibody concentration is too low, concentrate it by ultra-filtration in a centrifuge device. Note that in this process buffer changes can also be performed by repeated additions of the desired buffer with subsequent centrifugations.
- If the antibody concentration is too high, dilute in an amine-free buffer (e.g., PBS)
- My primary antibodies contain many additives. What should I do?
- For pre-treatment of the antibody before using Duolink® PLA Probemaker, we refer to standard procedures.
- To change buffer and/or to remove low molecular weight additives like azide or Trehalose, you can either dialyze against PBS or perform gel filtration on a spin column (Sephadex G25) equilibrated with PBS.
- To remove high molecular weight additives such as BSA or gelatin, we recommend purification with Protein A or Protein G. The antibody is eluted with low pH which might affect antibody activity. We recommend to immediately add a strong buffer of neutral pH. There is always a loss of antibody with this purification method.
- What dilution of the conjugated antibody should I use?
The working concentration of the antibody will be dependent mainly on the sensitivity of the antibody, the sample type and the sample pretreatment. Our general recommendation is to use a starting concentration that gives good results with IF or IHC. Sometimes, titration may be necessary. - Can I combine a directly conjugated primary antibody and a PLA probe?
Yes. It is possible to combine a directly conjugated antibody and a Duolink® PLA probe as long as one of them is PLUS and the other is MINUS and there is no species cross-reactivity (that is, the PLA probe should be directed against a species different from your directly conjugated primary antibody).
Additional Support
Contact us for further support.
When contacting us, please provide the following information:
- Description of your assay
- Images of your results
- Controls (positive/negative, biological/technical) that have been performed (if any)
- Previous IF/IHC results (if any)
Network error: Failed to fetch
Pour continuer à lire, veuillez vous connecter à votre compte ou en créer un.
Vous n'avez pas de compte ?