Ylide-functionalized Phosphines (YPhos)
Coupling Reactions Under Mild Conditions
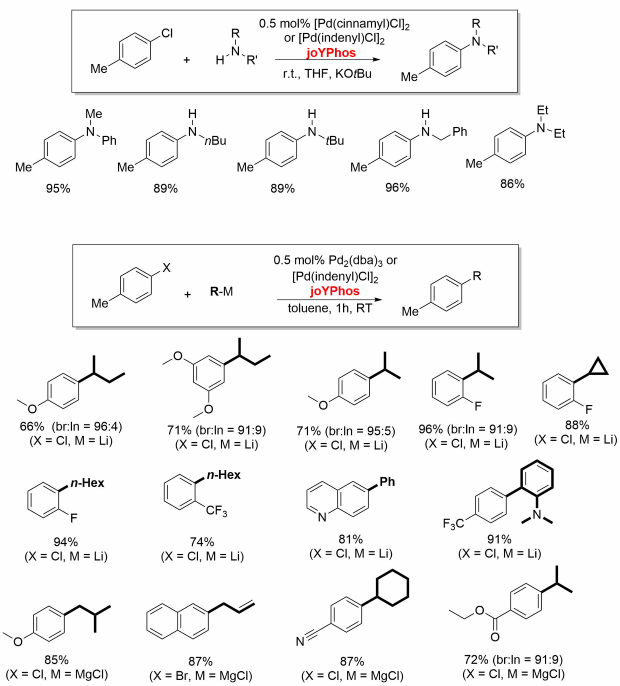
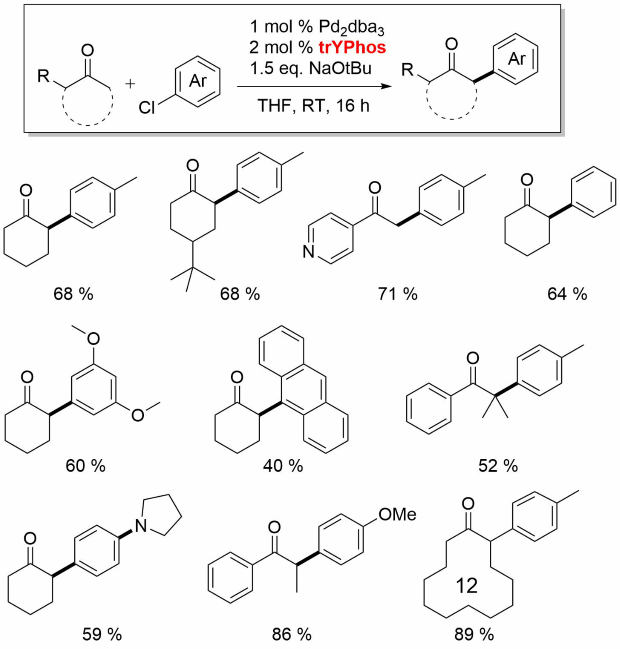
Professor Viktoria Gessner and coworkers from the Ruhr-University Bochum have developed a class of ylide-substituted phosphines (YPhos) which contain a bulky ylide-substituent directly bound at the phosphorus atom.[1] These ligands are especially electron-rich and thus allow for palladium catalyzed coupling reactions at remarkably mild reaction conditions. They enable the conversion of often challenging aryl chlorides in good yield with short reaction times. For example, keyPhos (No. 913294) and joYPhos (No. 912042) perform excellently in the Buchwald-Hartwig aminations at room temperature. JoYPhos also readily converts aryl halides (including chlorides) with organolithium, Grignard and zinc reagents. The strongest donor trYPhos (No. 913030) is best suited for challenging alpha-arylations.

Key Advantages of YPhos Ligands
- Facile coupling of aryl halides, especially aryl chlorides
- Mild reaction conditions
- High activity in C-N and C-C cross coupling reactions
- Coupling of organolithium, magnesium and zinc reagents
Representative Applications of YPhos Ligands
keYPhos with a methyl group in the ylide-backbone is a valuable ligand for the palladium catalyzed coupling of aryl chlorides with primary and secondary alkyl and aryl amines at room temperature. The ligand performs well with common palladium sources such as Pd2(dba)3, Pd(OAc)2, [Pd(allyl)Cl]2 or [Pd(cinamyl)Cl]2.[2,3]

JoYPhos featuring a phenyl group at the ylide substituent is also an excellent ligand for Buchwald-Hartwig aminations[4,5] as well as for C-C coupling reactions. It allows the previously unprecedented direct coupling of alkyllithium reagents with aryl chloride.[6]
In this series, trYPhos is the most electron-rich YPhos ligand and was found to be active for alpha-arylation of aliphatic cyclic ketones.[7]
References
Pour continuer à lire, veuillez vous connecter à votre compte ou en créer un.
Vous n'avez pas de compte ?