Ephedrine/Pseudoephedrine HCl in Oral Solution Analysis
Dean Duan
MilliporeSigma China Application Lab Shanghai, China
Chinese Pharmacopeia Method
Ephedrine hydrochloride and pseudoephedrine hydrochloride can be used to reduce cold, allergic rhinitis, rhinitis and sinusitis caused by nasal congestion symptoms and control bronchial asthma. The same compounds are also found in the Chinese traditional medicine Xiao’er Kechuanling oral solution.
This application focuses on the determination of ephedrine HCl and pseudoephedrine HCl. Oral solution samples were distilled to extract the ephedrine HCl and pseudoephedrine HCl. Samples were then filtered through Millex® PTFE syringe filters, prior to HPLC-UV analysis using a Purospher® STAR RP-18 endcapped HPLC column.
The limit of detection (LOD) and the limit of quantitation (LOQ) were 0.04 µg/kg and 0.12 µg/kg, respectively, for ephedrine, and 0.02 µg/kg and 0.06 µg/kg, respectively, for pseudoephedrine. The method can be used for determination of ephedrine HCl and pseudoephedrine HCl in Xiao’er Kechuanling oral solution.

Figure 1.Chemical structures of compounds used in the study.
Determination of Ephedrine HCl and Pseudoephedrine HCl in Xiao’er Kechuanling Oral Solution
Purospher® STAR RP-18e

Figure 2.Chromatogram of ephedrine HCl and pseudoephedrine HCl standard solution.

Figure 3.Chromatogram of in Xiao’er Kechuanling oral solution.
Figure 4.Chromatogram of solvent blank.
Specificity and Repeatability – Ephedrine and Pseudoephedrine
Specificity: Inject reference solution and determine the retention time of desired analyte in presence of other components like impurities. |
|---|
Calibration Data - Ephedrine
Linearity (area mAU*min), ephedrine HCl |
|---|
LOD and LOQ, ephedrine HCl |
|---|
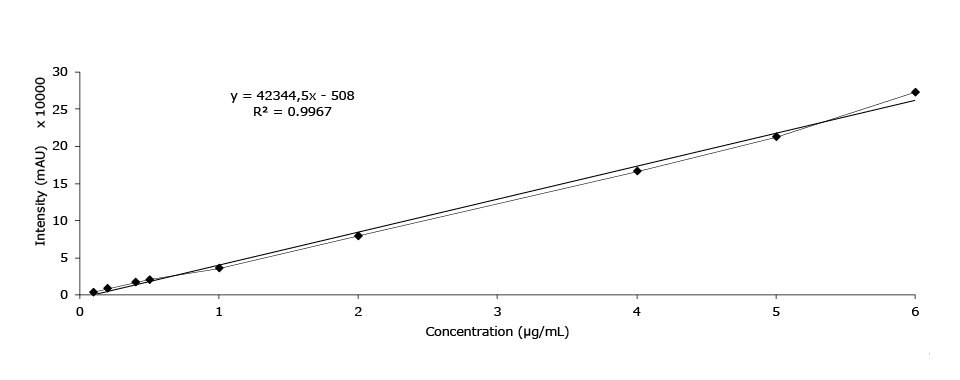
Figure 5.Calibration curve of ephedrine HCl.
Calibration Data - Pseudoephedrin
Linearity (area mAU*min), pseudoephedrine HCl |
|---|
4.2 LOD and LOQ - pseudoephedrine HCl |
|---|
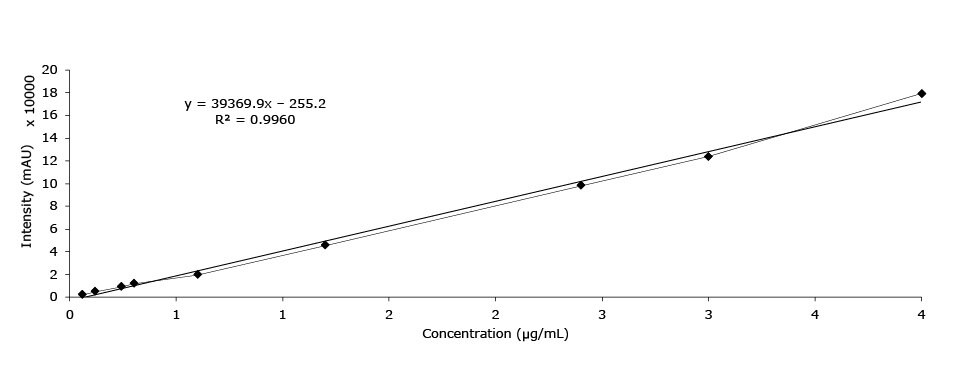
Figure 6.Calibration curve of pseudoephedrine HCl.
Conclusion
The method can be used for ephedrine HCl and pseudoephedrine HCl in Xiao’er Kechuanling oral solution.
Pour continuer à lire, veuillez vous connecter à votre compte ou en créer un.
Vous n'avez pas de compte ?