Organoids Generation and Phenotypic Screen on Millicell® Microwell Plates
An increasingly common tool used in biomedical research applications, organoids are 3D miniaturized models of organs that can be reproduced in vitro. Organoids are promising models for applications such as cancer research, chemotherapy development, and the development of personalized medicine.
Traditional organoids are cultured within a solidified extracellular matrix (ECM), which is often not reproducible. The organoids formed can have heterogeneous sizes and shapes and may be randomly placed in multiple positions and focal plans within the hydrogel. Millicell® Microwell 96-well plates standardize and overcome some of the challenges found in traditional organoid culture.

Figure 1. Traditional organoid culture (left) vs organoid culture in Millicell® Microwell 96-well plates (right).
Millicell® Microwell 96-well Plate Attributes
Millicell® Microwell plates are a ready-to-use platform for high throughput, scalable, and reliable organoid culture. These plates contain non-adherent microwells within the wells, increasing homogeneous organoid yield. Millicell® Microwell plates require little to no external solid ECM, each microwell contains a unique water-based polyethylene glycol (PEG) hydrogel. The PEG hydrogel allows for in-well cell seeding and assay development, and less diffraction during in-plate imaging.
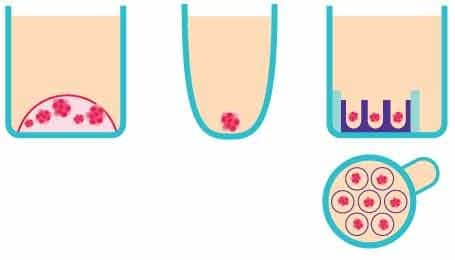
Figure 2.Schematic representation of the in vitro 3D organoid screening methods. From left to right, Matrigel®, a non-adherent surface, and Millicell® Microwell plates.
Our standard designs for different microwell sizes allow for scaled up organoid generation using Millicell® Microwell 96-well plates.
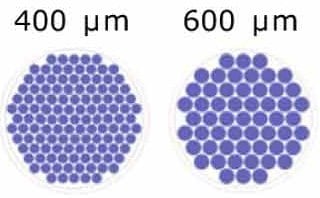
Figure 3. Schematic representation of the microwells/well for each microwell size.
Mouse intestinal organoids development and characterization on Millicell® Microwell plates
One of the first organoids to be described, mouse intestinal organoids are commonly used models for researchers studying cancer therapies, immunology, and microbiomics. We used Millicell® Microwell 400μm 96-well plates for the development and characterization of mouse intestinal organoids.
The organoids were monitored throughout seeding, growth, and imaging; 121 single organoids were developed over time in one well. They were found to be of homogeneous size and were confined to the microwells, facilitating imaging. The mouse intestinal organoids showed typical budding morphology after differentiation and the differentiated intestinal cells expressed specific intestinal markers.

Figure 4.Mouse intestinal organoids grown on Millicell® Microwell plates. A) 1 hour, B) 10 hours, C) 2 hours, D) 3 days, and E) 4 days after seeding.
Area of the organoids was quantified through imaging over time in Millicell® Microwell 96-well plates. The organoids were confined to a specific microcavity and were homogeneously sized in the first days 1-3 of growth before broadening as the organoids began budding.

Figure 5.Representative examples of mouse intestinal organoids grown on Millicell® Microwell plates on days A) 3; B) 5; and C) 8.
The organoids presented the budding phenotype that is typical for these intestinal organoids and contained differentiated cells that include enteroendocrine, eterocytes, Paneth, and goblet cells. Specific markers and epithelial polarity were monitored through immunofluorescence on glass imaging slides (Figure 6).
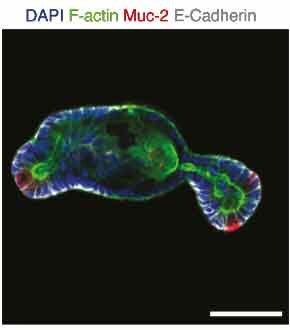
Figure 6.Protein expression of mucin-2 highlighting the presence of Goblet cells in organoids grown in microwell assays.
Organoid generation using Millicell® Microwell plates is scalable and generated organoids of similar size and shape.
CRC Organoid Phenotypic Screen on Millicell® Microwell plates
We performed an automated drug screen of 80 anti-cancer compounds on HCT-116 spheroids and patient-derived colorectal cancer (CRC) organoids using Millicell® Microwell 96-well plates. The response of the homogeneous microtissues to treatment was accessed by high content imaging phenotypic screens and over 250 phenotypic metrics were extracted.
When analyzing the phenotypes of the organoids grown on Millicell® Microwell 96-well plates, a previously unobserved phenotype was revealed during the administration of afuresertib. Here we demonstrate that Millicell® Microwell 96-well plates can be used to assess the effects of drugs and other compounds at a single-organoid level in a fully automated, high throughput screen.

Figure 6.CRC organoid drug screen workflow. A) Protocol for the compound screen of CRC organoids and HCT-116 spheroids on Millicell® Microwell 96-well plates, B) Schematic of automated (Hydrogel equilibrium, Cell Seeding, Medium changes & drug exposure and Analysis) vs manual (organoid dissociation) organoid generation, C) Quantification of the percentage of wells that contained a colony in manual vs automatic organoid generation.
The viability of the organoids was assessed after compound exposure and their phenotype was monitored through high-content imaging analysis. More than 250 metrics were extracted from each of the 40 organoids that were segmented per well and the efficacy of the drugs was determined through positive and negative controls. The screen showed high reproducibility, with an R2 value of 0.9 for spheroids and 0.79 for organoids.
In depth analysis of afuresertib on CRC organoids
Afuresertib is an inhibitor of serine/threonine protein kinase Akt (protein kinase B). It may inhibit tumor cell proliferation and induce tumor cell apoptosis through inhibiting Akt. Analysis of the 80 compounds that were used in this study showed that exposure to afuresertib led to strong phenotypes at subtoxic concentrations, including major swelling on the CRC organoids and not the HCT-116 spheroids. This is a phenotype that is not often monitored in classical viability analyses but can be easily seen using Millicell® Microwell 96-well plates.

Figure 7. CRC organoid screen of afuresertib. A) Wide-field and fluorescence images displaying organoid size in differing afuresertib concentrations, B) Organoid phenotypic response to increasing concentrations of afuresertib by microtissue area IC50=0.31μM, C) Organoid viability at increasing afuresertib concentrations IC50=10.67μM. Gambogic acid: GA.
CRC Organoid Phenotypic Screen Summary
The generation of CRC organoids and HCT-116 spheroids in Millicell® Microwell plates, from seeding to imaging readouts, was compatible with automatic liquid handling systems and high throughput screening applications. Millicell® Microwell plates also allowed for single-organoid analysis instead of population-based readouts, even with the generation of more than 70 organoids per well. This attribute helps researchers discover phenotypes that would otherwise go unnoticed.
The patient-derived organoids that were generated in Millicell® Microwell plates were found to closely model the corresponding tumor and can be used by researchers to study patient-specific responses to chemotherapies.
References
Pour continuer à lire, veuillez vous connecter à votre compte ou en créer un.
Vous n'avez pas de compte ?