Consistent, reliable, simple.
Improve your research with Strat-M® Transdermal Diffusion Membrane.
Strat-M® Transdermal Diffusion Membrane
Experience the unmatched predictability of Strat-M® membrane — synthetic, non-animal-based model for transdermal diffusion testing that is predictive of diffusion in human skin without lot-to-lot variability, safety, or storage limitations. How does the Strat-M® membrane mimic human skin? Watch this animation to find out and learn how the Strat-M® membrane can enable formulation optimization.
Designed for transdermal diffusion and safety testing of:
- Cosmetic Actives
- Formulations
- Personal Care Products
- Active Pharmaceutical Ingredients (API)
- Pesticides and Chemicals
BROAD CHEMICAL COMPATIBILITY
Pharmaceutical Work Flow
| API Screening |
|||
| ⮟ | |||
| Formulation Screening | |||
| ⮟ | |||
| Formulation Optimization | |||
| ⮟ | |||
| Clinical Development | |||
| ⮟ | |||
| Post-Clinical Manufacturing | |||
| ⮟ | |||
| Post-Market Activites | |||
Personal Care Work Flow
| 'Actives' Screening |
||
| ⮟ | ||
| Formulation & Development | ||
| ⮟ | ||
| Clinical Development | ||
| ⮟ | ||
| Post-Market Manufacturing |
| Use of skin or synthetic skin models |
We’ve engineered predictive performance into the structure and chemistry of the Strat-M® membrane. Looking at the pharmaceutical & personal care workflow, Strat-M® membrane can be used where biological models are traditionally used and is an appropriate model for screening compounds with diverse physiochemical properties.
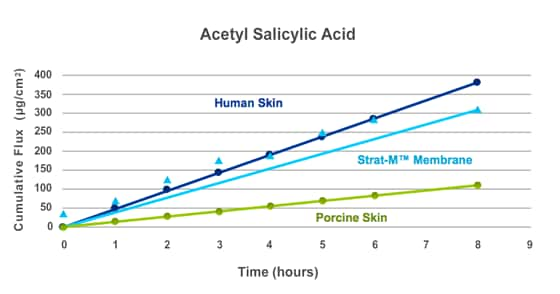
Sources - Human Cadaver Skin: International Journal of Pharmaceutics, 2006, vol 310, pg 31-36; Porcine Skin: International Journal of Pharmaceutics, 1999, vol 181, pg 255-263
STRAT-M® MEMBRANE CORRELATES BETTER TO HUMAN SKIN COMPARED WITH OTHER BIOLOGICAL MODELS
Many researchers are using animal skin as a surrogate for human skin in IVPT studies because human skin is either difficult to obtain or in many countries, it cannot be used for in vitro research. Pig, rat, or mouse skin are commonly used as surrogates for human skin.
Strat-M® membrane correlates better to human skin compared with some of these animal skin models. For acetylsalicylic acid (aspirin, an analgesic), high flux was measured through both human skin and Strat-M® membrane; however, flux was lower through porcine skin, indicating that Strat-M® membrane is a better match for human skin than animal skin models.
For more information about how Strat-M correlates to human skin vs other biological models, see our brochure.

STRAT-M® MEMBRANE CORRELATION DATA
Are you:
- Testing active compounds for transdermal diffusion?
- Optimizing your formulations?
- Using enhancers?
- Performing safety testing on your formulations?
Use our interactive Compound Correlation Tool to learn how well the Strat-M® membrane will work for your specific needs.
Related Product Resources
- Strat-M® Membrane for Sunscreen Formulation Testing
Strat-M synthetic membrane model is an alternative to human skin for testing the effectiveness of skin care actives.
- Strat-M® Transdermal Diffusion Membrane Performance
Strat-M® membrane has broad chemical compatibility and is an appropriate test model for compounds across a wide range of physiochemical properties.
- Chitosan-based Biomaterials
Chitin, a natural polysaccharide, is the second most abundant natural biopolymer in the world, after cellulose.
- Chem Compatibility of Materials in Millipore® Filtration
Filter compatibility data for membranes and housing materials, aiding in filtration product selection and use.
Strat-M® Membranes in Action: Predicting Transdermal Diffusion
We’ve compiled a sampling of peer-reviewed studies which cite Strat-M® membranes, detailing procedures for its use in skin diffusion testing.
References
Pour continuer à lire, veuillez vous connecter à votre compte ou en créer un.
Vous n'avez pas de compte ?
