Task-Specific Ionic Liquids
Roland St. Kalb1, Michael J. Kotschan1
Proionic Production of Ionic Substances GmbH, Leoben, Austria
Introduction
Task-specific ionic liquids (TSILs) for the selective liquid/liquid extraction of heavy metals from aqueous systems were first published by Robin D. Rogers et al. in the year 2001.1 Functionalized imidazolium cations with thioether, urea, or thiourea derivatized side chains act as metal-ligating moieties, whereas the PF6– anions provide the desired water immiscibility (Figure 1). Nernst distribution ratios were reported for Cd2+ and Hg2+ to be ≤380.
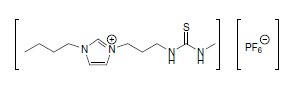
Figure 1.Thiourea derivatized Ionic Liquid
These ionic liquids were the first to contain specific functionalities to enable well defined chemical properties and therefore undoubtedly be called “designer solvents.”
However, these pioneering ionic liquids show some major drawbacks: the hexafluorophosphate anion is known to be quite unstable toward hydrolysis and produces toxic and corrosive HF or fluorides. The toxicity of the imidazolium cation is difficult to be estimated; an expensive toxicological study is risky. The disposal of these fluorous compounds is expensive and problematic. The synthesis at larger scale is complicated; starting materials are expensive.
Trioctylmethylammonium thiosalicylate (TOMATS): A Novel, High Performance, Ionic Liquid for the Extraction of Heavy Metals from Aqueous Solutions
To overcome the aforementioned drawbacks—especially in consideration of a possible industrial application at larger scales—and to enhance the performance, proionic Production of Ionic Substances GmbH developed a new Ionic Liquid in their laboratories: Trioctylmethylammonium thiosalicylate (“TOMATS,” Product No. 08354, Figure 2).
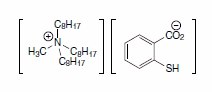
Figure 2.TOMATS
TOMATS contains no fluorine and is absolutely stable to any hydrolysis. It therefore does not release HF or fluorides, is not corrosive, and is much easier to dispose. The toxicity of the cation is known from common compounds like trioctylammonium chloride (a phase-transfer catalyst). The toxicity of the anion is known from thiosalicylic acid and its salts; they are found to be irritants. The distribution coefficients of heavy metals typically show values in the range of 1500 to more than 5000, which may be explained by the chelating effect of the ortho- positioned carboxylate group additional to the known formation of metal-thiolates. The synthesis of TOMATS is simple and can be done at industrial scales.
Application of TOMATS
Figure 3 shows the extraction of copper out of a blue-colored aqueous Cu2+-tetramine phase (left test tube). After adding the TOMATS Ionic Liquid and before shaking, nice diffusion zones can be seen (middle test tube), showing a copper-free, uncolored region and a dark copper-containing upper region. After shaking and waiting for the separation of the phases, all the copper is extracted into the upper phase, forming an organic, dark-colored copper compound (right test tube).

Figure 3.Extraction of Copper
Phase separation sometimes can last a long period of time because of the relative high viscosity of TOMATS of 1,500 mPa.s at 20 °C. This drawback can be overcome by either adding some percent of a water-immiscible organic solvent like ethyl acetate or dichloromethane (decreasing the viscosity down to hundred mPa.s), or by heating.
Phase separation can be optimized using a centrifuge and adding some sodium sulfate to the aqueous phase before shaking. If the aqueous phase still looks turbid, it can be filtered through a common membrane filter (μM pore size).
Characterization of TOMATS
Appearance: olive green, viscous liquid
Solubility:
- Soluble in alcohols, ethyl acetate, THF, acetonitrile, acetone, dichloromethane, DMSO
- nsoluble in water, hexane
Nernst distribution coefficients: 1 Cd2+ >1,500; Cu2+ >3,000; Pb2+, Hg2+ >5,000
Refractive index: nD20 = 1.5185
Leaching into aqueous phase: <0.5%
Density, Viscosity:
References
To continue reading please sign in or create an account.
Don't Have An Account?