Performing a Separation or Removal of Albumin with HiTrap® Blue HP and Blue Sepharose 6 Fast Flow
The same procedure can be used either to purify albumin or to remove albumin as a specific contaminant before or after other purification steps.
Albumin binds to Cibacron™ Blue F3G-A, a synthetic polycyclic dye that acts as an aromatic anionic ligand binding the albumin via electrostatic and/or hydrophobic interactions. Similar interactions are seen with coagulation factors, lipoproteins and interferon. Cibacron Blue F3G-A is linked to Sepharose to create Blue Sepharose affinity media.

Figure 1. Partial structure of Blue Sepharose Fast Flow and Blue Sepharose High Performance.
Use HiTrap® Blue HP 1 mL or 5 mL columns to remove host albumin from mammalian expression systems, or when the sample is known to contain high levels of albumin that may mask the visualization of other protein peaks seen by UV absorption.
Albumin can be a significant contaminant during the purification of immunoglobulins from ascites fluid, cell cultures or serum, chiefly because of its abundance in the original source material.
Cibacron Blue F3G-A also shows certain structural similarities to naturally occurring molecules, such as the cofactor NAD+, that enable it to bind strongly and specifically to a wide range of proteins including kinases, dehydrogenases and most other enzymes requiring adenylyl-containing cofactors (see page 73, NAD+-dependent dehydrogenases and ATP-dependent kinases).
* A convenient alternative to Blue Sepharose CL-6B, since rehydration is not required.
** See Appendix 4 to convert linear flow (cm/h) to volumetric flow rate. Maximum operating flow is calculated from measurement in a packed column with a bed height of 10 cm and i.d. of 5 cm.
Purification Examples
Figure 42 shows the use of HiTrap® Blue HP for purification of increasing amounts of human serum albumin. The process is easily scaled up by connecting several 1 ml or 5 mL HiTrap® columns in series.
Figure 43 shows the use of Blue Sepharose 6 Fast Flow for the separation of human serum albumin from interferon β.
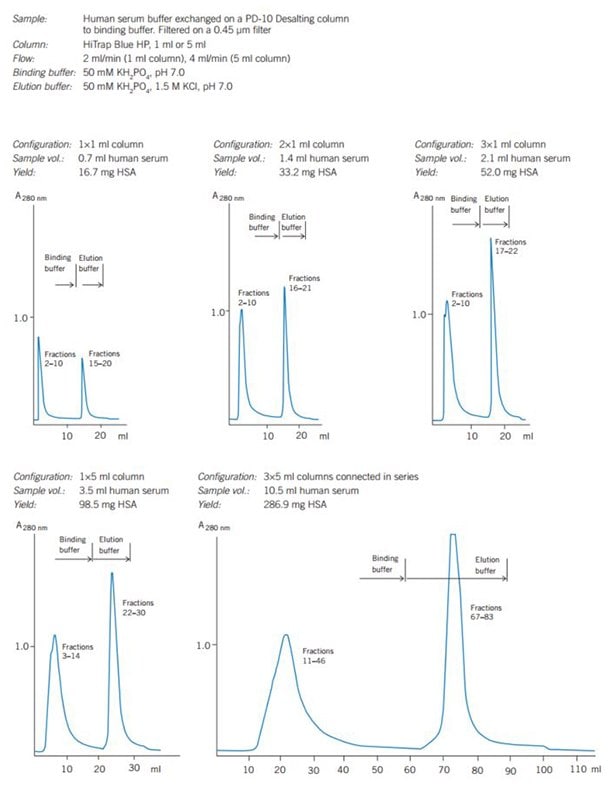
Figure 2. Scaling up on HiTrap® Blue HP gives predictable separations and quantitatively reproducible yields.
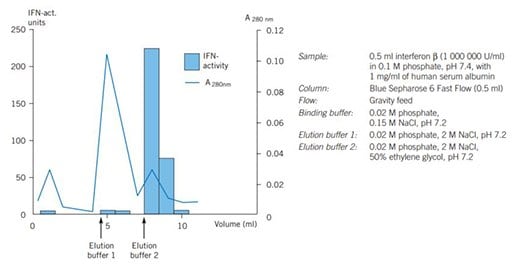
Figure 3. Purifcation of human serum albumin and interferon β on Blue Sepharose 6 Fast Flow.
In these examples elution is achieved by increasing the ionic strength of the buffer or changing the polarity of the buffer. Changing the pH of the buffer can also work, but the correct co-factor is preferable for the elution of specifically bound proteins.
Performing a Separation
Binding buffer: 50 mM KH2PO4, pH 7.0 or 20 mM sodium phosphate, pH 7.0
Elution buffer: 50 mM KH2PO4, 1.5 M KCl, pH 7.0 or 20 mM sodium phosphate, 2 M NaCl, pH 7.0
- Equilibrate the column with 5 column volumes of binding buffer.
- Apply the sample, using a syringe or a pump.
- Wash with 10 column volumes of binding buffer or until no material appears in the eluent (monitored by absorption at A280 nm).
- Elute with 5 column volumes of elution buffer. More may be required if the interaction is difficult to reverse.
Cleaning
Wash with 5 column volumes of high pH (0.1 M Tris-HCl, 0.5 M NaCl, pH 8.5) followed by low pH (0.1 M sodium acetate, 0.5 M NaCl, pH 4.5). Repeat 4–5 times. Re-equilibrate immediately with binding buffer.
Remove precipitated proteins with 4 column volumes of 0.1 M NaOH at a low flow rate, followed by washing with 3–4 column volumes of 70% ethanol or 2 M potassium thiocyanate. Alternatively, wash with 2 column volumes of 6 M guanidine hydrochloride. Re-equilibrate immediately with binding buffer.
Remove strongly hydrophobic proteins, lipoproteins and lipids by washing with 3–4 column volumes of up to 70% ethanol or 30% isopropanol. Alternatively, wash with 2 column volumes of detergent in a basic or acidic solution, e.g. 0.1% non-ionic detergent in 1 M acetic acid at a low flow rate, followed by 5 column volumes of 70% ethanol to remove residual detergent. Re-equilibrate immediately in binding buffer.
Media Characteristics |
|---|
* Long term refers to the pH interval over which the medium is stable over a long period of time without adverse effects on its subsequent chromatographic performance. Short term refers to the pH interval for regeneration, cleaning-in-place and sanitization procedures.
Chemical Stability
Stable in all commonly used aqueous buffers, 70% ethanol, 8 M urea and 6 M guanidine hydrochloride.
Storage
Wash media and columns with 20% ethanol (use approximately 5 column volumes for packed media) and store at +4 to +8 °C.
To continue reading please sign in or create an account.
Don't Have An Account?