All Photos(2)
About This Item
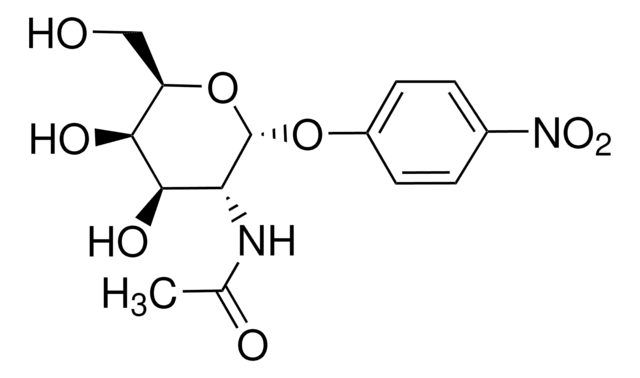
Empirical Formula (Hill Notation):
C5H10N2O · HCl
CAS Number:
Molecular Weight:
150.61
Beilstein:
3693546
EC Number:
MDL number:
UNSPSC Code:
12352200
PubChem Substance ID:
NACRES:
NA.26
Recommended Products
form
powder
SMILES string
Cl.NC(=O)[C@@H]1CCCN1
InChI
1S/C5H10N2O.ClH/c6-5(8)4-2-1-3-7-4;/h4,7H,1-3H2,(H2,6,8);1H/t4-;/m0./s1
InChI key
CSKSDAVTCKIENY-WCCKRBBISA-N
Looking for similar products? Visit Product Comparison Guide
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Martin G Schmid et al.
Analytical and bioanalytical chemistry, 400(8), 2305-2316 (2011-02-15)
This article gives a short overview of the application of the principle of chiral ligand-exchange in HPLC, CE, and CEC. Since its introduction by Davankov, more than thousand articles have appeared in this field. Citing all these papers would extend
Hizuru Nakajima et al.
Analytical sciences : the international journal of the Japan Society for Analytical Chemistry, 21(1), 67-71 (2005-01-29)
The chiral separation of amino acid derivatives by ligand-exchange electrophoresis in a microchannel chip was performed for the first time. A Cu(II) complex with L-prolinamide was used as a chiral selector. The migration behaviors of eleven NBD-DL-amino acids were investigated
Patrycja Puchalska et al.
Electrophoresis, 31(9), 1517-1520 (2010-04-28)
A new chiral stationary phase based on continuous bed (CB) technology using L-prolinamide as a chiral selector was prepared. Its ability for enantioseparation of amino acids and alpha-hydroxy acids by ligand-exchange CEC was compared with that of a CB containing
Li Qi et al.
Journal of separation science, 32(18), 3209-3214 (2009-08-26)
A novel method of chiral ligand-exchange CE was developed with L-amino acylamides as a chiral ligand and zinc(II) as a central ion. It has been demonstrated that these chiral complexes, such as Zn(II)-L-alaninamide, Zn(II)-L-prolinamide, and Zn(II)-L-phenylalaninamide, are suitable for use
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service