All Photos(2)
About This Item
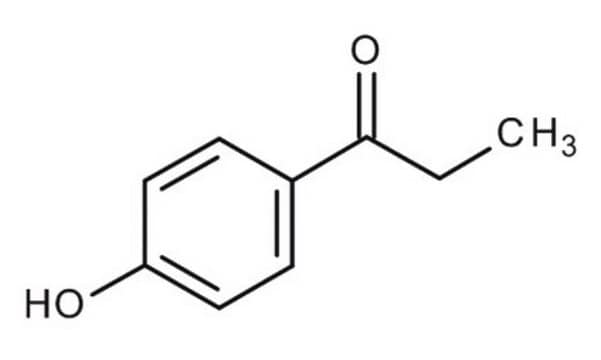
Linear Formula:
HOC6H4COC2H5
CAS Number:
Molecular Weight:
150.17
Beilstein:
907511
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
mp
147.5-148.5 °C (lit.)
SMILES string
CCC(=O)c1ccc(O)cc1
InChI
1S/C9H10O2/c1-2-9(11)7-3-5-8(10)6-4-7/h3-6,10H,2H2,1H3
InChI key
RARSHUDCJQSEFJ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Morio Yoshimura et al.
Chemical & pharmaceutical bulletin, 65(9), 878-882 (2017-09-05)
Mousouchiku extract is prepared from the bamboo-sheath of Phyllostachys heterocycla MITF. (Poaceae), and is registered as a food manufacturing agent in the List of Existing Food Additives in Japan. This study describes the chromatographic evaluation of characteristic components of this
R Cizmáriková et al.
Ceskoslovenska farmacie, 42(2), 82-85 (1993-04-01)
Within the relationship of the structure and effect of new beta-adrenolytic agents derivatived from p-hydroxyacetophenone and p-hydroxypropiophenone with a propoxymethyl group in the lipophilic part of the molecule and with a propanamine, a butanamine and a pyrrolidine in the side-chain
p-Hydroxypropiophenone effects on azo dye-induced alterations in mouse hepatic cells: light and electron microscopic study.
N J Unakar
Journal of the National Cancer Institute, 44(4), 873-891 (1970-04-01)
Zack E Bryant et al.
Bioorganic & medicinal chemistry letters, 21(3), 912-915 (2011-01-14)
A series of ethacrynic acid analogues, lacking the α,β-unsaturated carbonyl unit, was synthesized and subsequently evaluated for their ability to inhibit the migration of human breast cancer cells, Hs578Ts(i)8 as well as of human prostate cancer cells, C4-2B. These cell
R Cizmáriková et al.
Ceska a Slovenska farmacie : casopis Ceske farmaceuticke spolecnosti a Slovenske farmaceuticke spolecnosti, 43(5), 226-228 (1994-10-01)
The present paper carries out the pharmacological evaluation of 4-(2-hydroxy-3-isopropylaminopropoxy)-3-(alkoxymethyl) propiophenones with an ethoxy, propoxy and butoxy-group, whose structures are typical of the blockers of beta-adrenergic receptors. In the above-mentioned compounds the anticalcium effect on the frequency and the amplitude
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service