All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C7H8O3
CAS Number:
Molecular Weight:
140.14
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
liquid
refractive index
n20/D 1.46 (lit.)
bp
93-95 °C/35 mmHg (lit.)
density
1.038 g/mL at 25 °C (lit.)
storage temp.
2-8°C
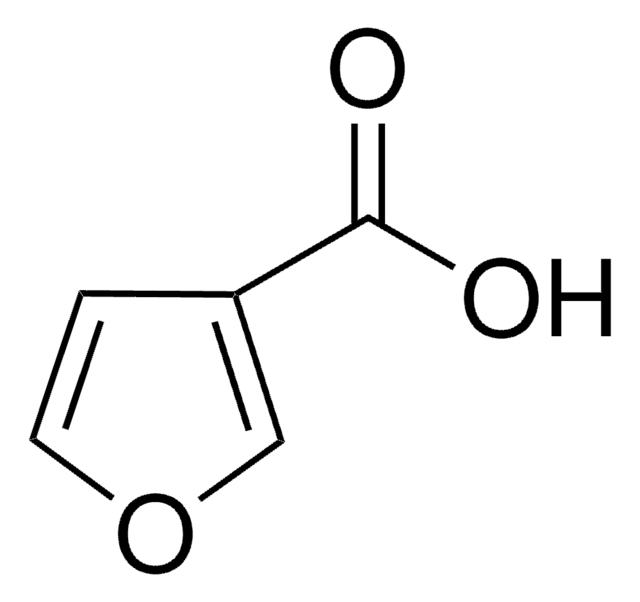
SMILES string
CCOC(=O)c1ccoc1
InChI
1S/C7H8O3/c1-2-10-7(8)6-3-4-9-5-6/h3-5H,2H2,1H3
InChI key
LOFDXZJSDVCYAS-UHFFFAOYSA-N
General description
Ethyl 3-furoate undergoes regioselective palladium(0)-catalyzed arylation reaction with aryl bromides.
Application
Ethyl 3-furoate was used as starting reagent for the synthesis of ethyl 2,3-bis(trifluoromethyl)-7-oxabicyclo[2,2,1]hepta-2,5-diene-5-carboxylate and 4-(1-hydroxy-1-methyl-ethyl)-furan-2-sulfonamide.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Flam. Liq. 3
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
138.2 °F - closed cup
Flash Point(C)
59 °C - closed cup
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Novel Synthesis of 1-(1, 2, 3, 5, 6, 7-Hexahydro-s-indacen-4-yl)-3-[4-(1-hydroxy-1-methyl-ethyl)-furan-2-sulfonyl] urea, an Anti-inflammatory Agent.
Urban FJ, et al.
Synthetic Communications, 33(12), 2029-2043 (2003)
Some reactions of 3, 4-bis (trifluoromethyl) furan and its precursor, 2, 3-bis (trifluoromethyl)-7-oxabicyclo [2, 2, 1] hepta-2, 5-diene: novel isocoumarin formation from thermal reaction of the furan with ethyl propynoate.
Abubakar AB, et al.
Journal of Fluorine Chemistry, 56(3), 359-371 (1992)
Bobby Glover et al.
Organic letters, 5(3), 301-304 (2003-01-31)
[reaction: see text] The regioselective palladium(0)-catalyzed arylation of 3-furoate and 3-thiophenecarboxylate esters with aryl bromides is described. Conditions were developed that allow for the selective synthesis of either 2-aryl or 5-aryl products.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service