Near-infrared (NIR) Fluorescence Dyes
Tracy 645 and Tracy 652
Red-emitting fluorescent dyes with long-wavelength markers for use in the visible/near-infrared (NIR) region are useful in life science applications, such as antibody and protein labeling.
Tracy 645 and Tracy 652 (Table 1) are proprietary dyes developed to meet the need for NIR fluorescent dyes. The advantages for using Tracy dyes are as follows:
- Able to retain chemical stability of the activated N-hydroxysuccinimide (NHS) ester under protein labeling conditions (basic pH, which prevents dye decomposition or hydrolysis that could result in poor labeling efficiency).
- Photostable.
- Soluble in aqueous labeling media as the activated NHS ester, reducing precipitation that leads to inefficient protein labeling.
- Flexible for use in typical protein labeling protocols.
The decrease in background fluorescence results from both a dramatic reduction of Rayleigh and Raman scattering by shifting to the longer wavelength excitation and from the diminished autofluorescence of sample impurities in this region.
Several major advantages of the red-emitting fluorescent dyes over conventional shorter wavelength ones make the Tracy dyes highly attractive for cellular and molecular biology or bioanalytical studies.1-3
- Reduced background improves sensitivity.
- Strong fluorescent signal of labeled proteins at various dye/protein ratios.
- Low-cost, dependable laser diodes may be used as excitation sources instead of short-lived, expensive gas lasers.
Tracy 652 and Tracy 645 surpass conventional fluorescent labels, such as Cy® 5, in all of the above criteria (Figure 1). Other, more modern labels of the corresponding type are also deficient in one or more of the above criteria.
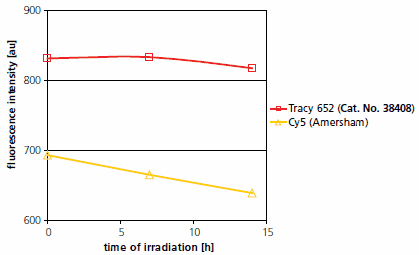
Figure 1.Photostability of Tracy 652 dye and Cy® 5.
Irradiation of each 1mM solutions in methanol in a white Pyrex flask by a 375 W white light lamp; distance from lamp to probe was 20 cm; probe maintained at room temperature; measured at λex,max and λem,max of each dye.
Investigation of optimal dye/protein (D/P) ratio of antibody conjugates of Tracy dyes showed a maximum emission intensity at ~4:1 D/P.
Secondary antibody conjugates with Tracy dyes are available for immunostaining applications observed by fluorescence microscopy (Figure 2).

Figure 2.Confocal microscopic image (CLSM) of a rat stomach section after immunohistological staining (2-channel mode). Green channel: staining of cytokeratin. Primary antibody: anti-cytokeratin from rabbit; secondary antibody: anti-rabbit IgG labeled with Atto 488 dye (λex 488 nm). Red channel: Staining of actin. Primary antibody: anti-actin from mouse; secondary antibody: anti-mouse IgG labeled with Tracy 645 dye (λex 543 nm).
Additionally, NTA (nitrilo-triacetate)-Tracy derivatives that selectively detect His-tagged proteins on solid surfaces such as gels or Western blots are also available in our catalog (Figure 3).

Figure 3.His-tagged p38-MAPK protein (500 ng–25 ng) was separated on a 4-20% Tris-Glycine SDS-PAGE gel. The gel was fixed overnight in 40% ethanol/10% acetic acid, washed in water, and incubated with Ni-NTA-Tracy 652 (1:1000) in the dark. The gel was washed and then imaged using a FLA-3000 Fuji® laser scanner with 633 nm excitation and a 675 nm emission filter. Ni-NTA-Tracy 652 (λex 652 nm, λem 677 nm) is excited in the red region of the spectrum.
The key advantages of this His-tagged protein detection method as compared to classical chemiluminescence-based detection on Western blots are the simplicity and efficiency of the protocol, but at the cost of some sensitivity.
Tracy 652 has been applied in two-color multiplex immunoblotting: Protein 1 and 2 in amounts ranging from 5 to 500 ng were separated on a 4-20% Tris-Glycine SDS-PAGE gel and then transferred to a low-fluorescence PVDF membrane. The membrane was blocked with 5% BSA in PBS, incubated with antibodies to protein 1 and 2 respectively, and probed with the corresponding secondary antibodies conjugated to fluorescent dyes Atto 550 (green) and Tracy 652 (red) (Figure 4).

Figure 4.Immunoblot detection of Protein 1 and Protein 2 using two primary antibodies and two anti-IgG dye conjugates. Sequential imaging with a FLA-3000 Fuij® laser scanner. First image for Atto 550: λex 532 nm with 580 nm emission filter. Second image for Tracy 652: λex 633 nm with 675 nm emission filter.
Para continuar lendo, faça login ou crie uma conta.
Ainda não tem uma conta?