860455P
Avanti
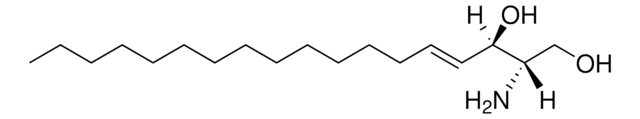
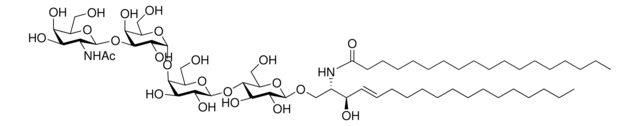
N-C12-deoxysphingosine
N-lauroyl-1-deoxysphingosine (m18:1/12:0), powder
Sinônimo(s):
N-dodecanoyl-1-deoxysphing-4-enine (m18:1/12:0); N-C12-1-deoxyCer; 110992
Faça loginpara ver os preços organizacionais e de contrato
About This Item
Fórmula empírica (Notação de Hill):
C30H59NO2
Número CAS:
Peso molecular:
465.79
Código UNSPSC:
12352211
NACRES:
NA.25
Produtos recomendados
Ensaio
>99% (TLC)
forma
powder
embalagem
pkg of 1 × 1 mg (860455P-1mg)
pkg of 1 × 5 mg (860455P-5mg)
fabricante/nome comercial
Avanti Research™ - A Croda Brand 860455P
tipo de lipídio
sphingolipids
bioactive lipids
Condições de expedição
dry ice
temperatura de armazenamento
−20°C
Descrição geral
N-C12-deoxysphingosine, also known as 1-deoxydihydroceramide (1-deoxyDHCer) is, the N-acylated form of 1-deoxysphinganine, a potent inhibitor of sphingolipid metabolism.
Aplicação
N-C12-deoxysphingosine has been used as a standard in the quantitation of atypical sphingoid bases in biological samples by reverse-phase liquid chromatography coupled to electrospray ionization tandem mass spectrometry (LC-MS/MS).
Embalagem
5 mL Amber Glass Screw Cap Vial (860455P-1mg)
5 mL Amber Glass Screw Cap Vial (860455P-5mg)
Informações legais
Avanti Research is a trademark of Avanti Polar Lipids, LLC
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Certificados de análise (COA)
Busque Certificados de análise (COA) digitando o Número do Lote do produto. Os números de lote e remessa podem ser encontrados no rótulo de um produto após a palavra “Lot” ou “Batch”.
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Nicholas C Zitomer et al.
The Journal of biological chemistry, 284(8), 4786-4795 (2008-12-20)
Fumonisin B(1) (FB(1)) is a mycotoxin that inhibits ceramide synthases (CerS) and causes kidney and liver toxicity and other disease. Inhibition of CerS by FB(1) increases sphinganine (Sa), Sa 1-phosphate, and a previously unidentified metabolite. Analysis of the latter by
Junliang Wan et al.
Journal of agricultural and food chemistry, 67(46), 12953-12961 (2019-10-23)
Most common sphingolipids are comprised of "typical" sphingoid bases (sphinganine, sphingosine, and structurally related compounds) and are produced via the condensation of l-serine with a fatty acyl-CoA by serine palmitoyltransferase. Some organisms, including mammals, also produce "atypical" sphingoid bases that
Terina N Martinez et al.
Molecular neurodegeneration, 7, 45-45 (2012-09-15)
Dopaminergic (DA) neurons in the ventral midbrain selectively degenerate in Parkinson's disease (PD) in part because their oxidative environment in the substantia nigra (SN) may render them vulnerable to neuroinflammatory stimuli. Chronic inhibition of soluble Tumor Necrosis Factor (TNF) with
Sarah T Pruett et al.
Journal of lipid research, 49(8), 1621-1639 (2008-05-24)
"Sphingosin" was first described by J. L. W. Thudichum in 1884 and structurally characterized as 2S,3R,4E-2-aminooctadec-4-ene-1,3-diol in 1947 by Herb Carter, who also proposed the designation of "lipides derived from sphingosine as sphingolipides." This category of amino alcohols is now
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica