Viral RNA Purification Protocol Using GenElute™ Mammalian Total RNA Purification Kits
For Research Use Only; not for use in diagnostic procedures. This protocol is relevant for catalog numbers RTN70 and RTN350.
The GenElute™ Mammalian Total RNA Purification Kit provides a rapid method for the isolation and purification of total RNA from viruses such as the 2019 Novel Coronavirus (SARS-CoV-2), which causes COVID-19 disease. Purification is based on spin column chromatography using a silica resin as the separation matrix. The following protocol is recommended for purification of viral RNA from cell-free samples including sera, plasma, and nasal and throat swabs.
Reagent Preparation
Required materials:
- GenElute™ Mammalian Total RNA Purification Kit (RTN70 or RTN350)
- 96-100% ethanol (non-denatured)
- RNase-free water
- Poly-A carrier RNA
- Microcentrifuge
- Sterile, RNAse/DNase-free pipette tips and tubes
Before you begin:
- Clean the workspace
- Prepare Poly-A carrier RNA
- Resuspend lyophilized carrier RNA with lysis buffer to a concentration of 1 µg/µL and divide into 20 µL aliquots. Store aliquots frozen at -20 °C. Avoid freeze-thaw.
- Prepare lysis buffer
- Determine how much lysis buffer you will need. Use 500 µL lysis buffer for each 120 µL plasma sample. Samples may need to be concentrated.
- Add 10 µL/mL β-mercaptoethanol (BME) to lysis buffer
- Add 10 µL/mL carrier RNA to lysis buffer
- It may be necessary to adjust the amount of carrier RNA for your application
- Vortex lysis buffer to mix
RNA Extraction Protocol for Serum and Plasma Samples
- Pipette 500 µL of prepared lysis buffer containing β-mercaptoethanol and carrier RNA into a 1.5 mL RNAse-free microcentrifuge tube (not provided).
- If sample volume is larger than 120 µL, increase the amount of lysis buffer proportionately.
- Add 120 µL plasma or cell-free fluid. Mix by pulse-vortexing for 10 seconds.
- Spin tubes briefly at low speed.
- Incubate at room temperature for 5 minutes.
- Add 500 µL of 100% ethanol. Mix by pulse-vortexing for 10 seconds.
- Do not use denatured alcohol
- If the sample volume is greater than 120 µL, increase ethanol volume proportionately.
- Proceed to viral RNA purification step.
RNA Extraction Protocol for Nasal or Throat Swabs
- Add 600 µL of prepared lysis buffer containing β-mercaptoethanol and carrier RNA into an RNase-free microcentrifuge tube.
- Gently brush a sterile, single-use cotton swab inside the nose or mouth of the subject.
- Using sterile techniques cut the cotton tip where the nasal or throat cells were collected and place into the microcentrifuge tube containing the lysis buffer.
- Close the tube.
- Vortex gently and incubate for 5 minutes at room temperature.
- Using a pipette, transfer the lysate into another RNase-free microcentrifuge tube.
- Note the volume of the lysate.
- Add an equal volume of 70% ethanol to the lysate volume collected (100 µL of ethanol is added to every 100 µL of lysate).
- Vortex to mix.
- Proceed to viral RNA purification step.
Viral RNA Purification
- Pipette up to 700 µL of sample/ethanol mixture into binding column and spin at 14,000 RPM for 15 seconds.
- Carefully discard flow through and repeat above step as needed for entire sample.
- Add 500 µL of Wash Buffer 1 to column and spin at 14,000 RPM for 15 seconds.
- Carefully move column to a new collection tube (provided) and label.
- Add 500 µL of Wash Buffer 2 to column and spin at 14,000 RPM for 15 seconds.
- Carefully discard flow through and repeat above step.
- Carefully discard flow through and spin at maximum speed for 2 minutes to dry column. Trace amounts of ethanol will have a negative effect on downstream processes.
- Move column to a new collection tube and label appropriately.
- Add 50 µL of elution buffer and incubate at room temperature for 5 minutes.
- Spin at 14,000 RPM for 1 minute.
- Use eluted RNA immediately or store at -80 °C.
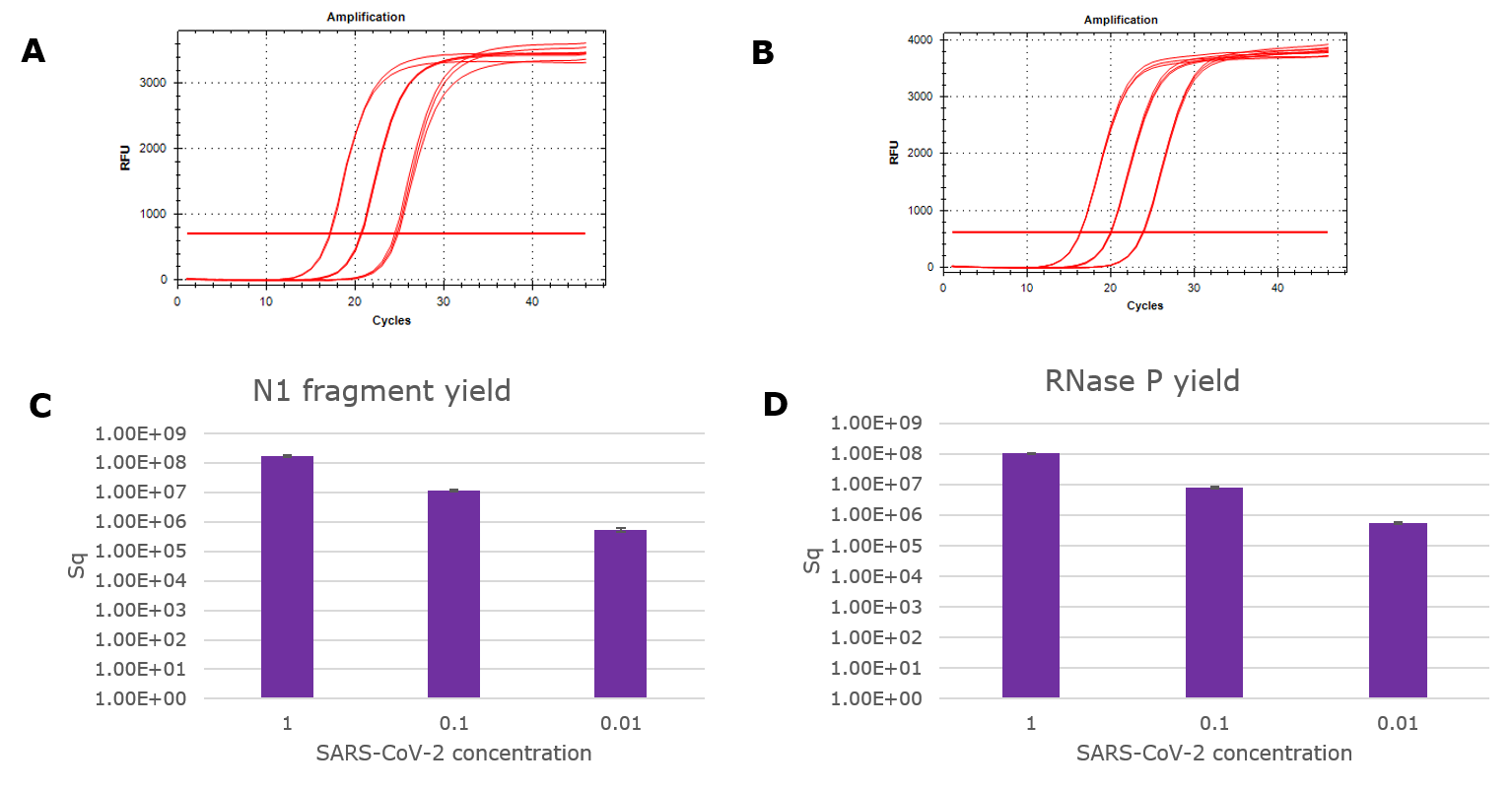
Figure 1.Yield of viral RNA purified with GenElute™ Mammalian Total RNA Purification Kit. An Armored RNA SARS-CoV-2 panel was serially diluted in Universal Transport Media and purified according to the above protocol. Viral RNA fragments were detected with TaqMan RT-qPCR kit (Norgen). Amplification curves from the N1 primer/ probe set (A) and the RNase P primer/ probe set (B) were quantified using standard curves to determine RNA yield (C and D).
To continue reading please sign in or create an account.
Don't Have An Account?