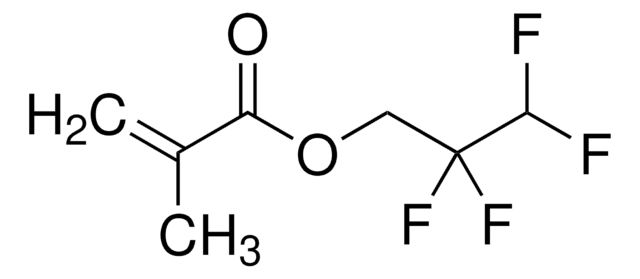
741108
Pentafluorophenyl methacrylate
contains MEHQ as inhibitor, 95%
Synonym(s):
Methacrylic acid, pentafluorophenyl ester
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C10H5F5O2
CAS Number:
Molecular Weight:
252.14
MDL number:
UNSPSC Code:
12162002
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
Assay
95%
form
liquid
contains
MEHQ as inhibitor
refractive index
n20/D 1.438
density
1.394 g/mL at 25 °C
storage temp.
2-8°C
SMILES string
CC(=C)C(=O)Oc1c(F)c(F)c(F)c(F)c1F
InChI
1S/C10H5F5O2/c1-3(2)10(16)17-9-7(14)5(12)4(11)6(13)8(9)15/h1H2,2H3
InChI key
NIJWSVFNELSKMF-UHFFFAOYSA-N
Related Categories
Application
The PFP unit acts as an activated ester for coupling (esterification/amidation) reactions. This is also a monomer for low refractive index polymers (n ~1.40).
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
172.0 °F
Flash Point(C)
77.8 °C
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
[Copolymerization of methyl methacrylate with pentafluorophenyl methacrylate and physical characteristics of the polymers (author's transl)].
Y Kadoma et al.
Iyo Kizai Kenkyujo hokoku. Reports of the Institute for Medical and Dental Engineering, Tokyo Medical and Dental University, 15, 23-29 (1981-01-01)
L Francesch et al.
Langmuir : the ACS journal of surfaces and colloids, 23(7), 3927-3931 (2007-03-07)
Pulsed-plasma polymerization has been used to deposit ultrathin layers of pentafluorophenyl methacrylate by using low duty cycles and low power input. The monomer structure can be retained such that the chemical reactivity of the active ester group could be studied
Nikhil K Singha et al.
Biomacromolecules, 12(8), 2908-2913 (2011-07-08)
Herein the concept of tandem postpolymerization modification as a versatile route to synthesize well-defined, highly functionalized polymers is introduced. Poly(pentafluorophenyl methacrylate) obtained by atom transfer radical polymerization was first modified with allylamine, which displaces the active ester to give well-defined
Claudia Battistella et al.
Biomacromolecules, 18(6), 1855-1865 (2017-04-15)
Inhibition of P-glycoprotein (P-gp) transporter is an attractive approach for the reversion of cancer-associated multidrug resistance (MDR). Poly(N-(2-hydroxypropyl) methacrylamide) (PHPMA)-based carriers that are able to release the anticancer drug doxorubicin in the lysosomes have shown promise to reduce P-gp mediated
Luis Duque et al.
Biomacromolecules, 11(10), 2818-2823 (2010-09-14)
Thin films of plasma polymerized pentafluorophenyl methacrylate (pp-PFM) offer highly reactive ester groups throughout the structure of the film that allow for subsequent reactions with different aminated reagents and biological molecules. The present paper follows on from previous work on
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service





![[2-(Methacryloyloxy)ethyl]dimethyl-(3-sulfopropyl)ammonium hydroxide 95%](/deepweb/assets/sigmaaldrich/product/structures/217/219/73c91e1c-0ee4-4b3d-bead-a6dc3d09d1da/640/73c91e1c-0ee4-4b3d-bead-a6dc3d09d1da.png)