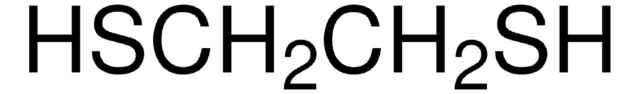8.00795
1,2-Ethanedithiol
for synthesis
Synonym(s):
1,2-Ethanedithiol
About This Item
Recommended Products
vapor pressure
6.4 hPa ( 20 °C)
Quality Level
Assay
≥99.0% (GC)
form
liquid
potency
120 mg/kg LD50, oral (Rat)
197 mg/kg LD50, skin (Rabbit)
mp
-41 °C
transition temp
flash point 50 °C
density
1.12 g/cm3 at 20 °C
storage temp.
2-30°C
InChI
1S/C2H6S2/c3-1-2-4/h3-4H,1-2H2
InChI key
VYMPLPIFKRHAAC-UHFFFAOYSA-N
Application
- Aryl thiols via copper-catalyzed single-step reaction with aryl halides.
- 1,3-Dithiolanes via Lewis acid-catalyzed chemoselective condensation with carbonyl compounds.
- Polyalkylene sulfides via polycondensation with dibromides in the presence of NaH.
Analysis Note
Density (d 20 °C/ 4 °C): 1.123 - 1.126
Identity (IR): passes test
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 2 Dermal - Acute Tox. 2 Inhalation - Acute Tox. 3 Oral - Flam. Liq. 3
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
111.2 °F - closed cup
Flash Point(C)
44 °C - closed cup
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Protocols
Fmoc resin cleavage and deprotection are crucial steps for peptide synthesis, yielding the desired peptide after resin detachment.
Fmoc resin cleavage and deprotection are crucial steps for peptide synthesis, yielding the desired peptide after resin detachment.
Fmoc resin cleavage and deprotection are crucial steps for peptide synthesis, yielding the desired peptide after resin detachment.
Fmoc resin cleavage and deprotection are crucial steps for peptide synthesis, yielding the desired peptide after resin detachment.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service