All Photos(1)
About This Item
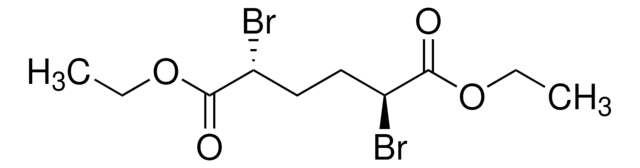
Linear Formula:
BrC(CH3)2COC(CH3)2Br
CAS Number:
Molecular Weight:
271.98
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
form
liquid
refractive index
n20/D 1.506 (lit.)
bp
89-91 °C/13 mmHg (lit.)
density
1.61 g/mL at 25 °C (lit.)
SMILES string
CC(C)(Br)C(=O)C(C)(C)Br
InChI
1S/C7H12Br2O/c1-6(2,8)5(10)7(3,4)9/h1-4H3
InChI key
SWSOAIMZWLDPST-UHFFFAOYSA-N
Related Categories
General description
2,4-Dibromo-2,4-dimethyl-3-pentanone is an α,α′-dibromoketone. Electroreduction of 2,4-dibromo-2,4-dimethyl-3-pentanone in polar aprotic solvent has been reported. Electrochemical reduction of 2,4-dibromo-2,4-dimethyl-3-pentanone in acetic acid and sodium actetate yields α-acetoxy ketones. 2,4-Dibromo-2,4-dimethyl-3-pentanone reacts with lithium dimethylcuprate(I) in diethyl ether at -78°C to yield 2,2,4-trimethyl-3-pentanone (an α methyl ketone).
Application
2,4-Dibromo-2,4-dimethyl-3-pentanone may be used in the synthesis of 2-dimethylamino-4-methylene-1,3-dioxolanes, via debromination using zinc-copper couple in dimethylformamide and dimethylacetamide.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
163.4 °F - closed cup
Flash Point(C)
73 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Electrochemical reduction of. alpha.,. alpha.'-dibromo ketones in acetic acid. Convenient synthetic route to highly branched. alpha.-acetoxy ketones.
Fry AJ and O'Dea JJ.
The Journal of Organic Chemistry, 40(25), 3625-3631 (1975)
Debromination of. alpha.,. alpha. 1-dibromo ketones with a zinc-copper couple in dimethylformamide and dimethylacetamide. New reaction yielding 2-dimethylamino-4-methylene-1, 3-dioxolanes.
Hoffmann HMR, et al.
Journal of the American Chemical Society, 94(9), 3201-3204 (1972)
Reaction of. alpha.,. alpha.'-dibromo ketones with organocopper reagents. New method for. alpha. alkylation of ketones.
Posner GH, et al.
Journal of the American Chemical Society, 95(9), 3076-3077 (1973)
Electroreduction of. alpha.,. alpha.'-dibromoketones. 2, 4-Dibromo-2, 4-dimethyl-3-pentanone.
Dirlam JP, et al.
Journal of the American Chemical Society, 94(1), 240-245 (1972)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service