46453
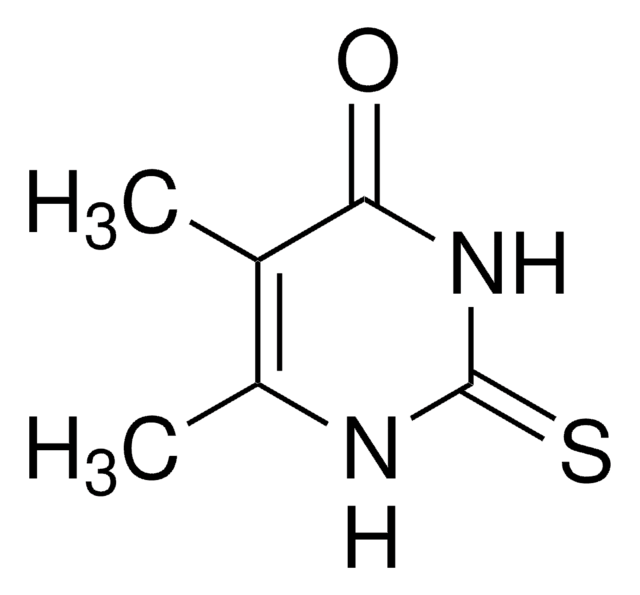
6-Methyl-2-thiouracil
VETRANAL®, analytical standard
Synonym(s):
4-Hydroxy-2-mercapto 6-methylpyrimidine, MZU
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C5H6N2OS
CAS Number:
Molecular Weight:
142.18
Beilstein:
115648
EC Number:
MDL number:
UNSPSC Code:
41116107
PubChem Substance ID:
NACRES:
NA.24
Recommended Products
grade
analytical standard
product line
VETRANAL®
shelf life
limited shelf life, expiry date on the label
technique(s)
HPLC: suitable
gas chromatography (GC): suitable
mp
~330 °C (dec.) (lit.)
application(s)
clinical testing
format
neat
SMILES string
CC1=CC(=O)NC(=S)N1
InChI
1S/C5H6N2OS/c1-3-2-4(8)7-5(9)6-3/h2H,1H3,(H2,6,7,8,9)
InChI key
HWGBHCRJGXAGEU-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Refer to the product′s Certificate of Analysis for more information on a suitable instrument technique. Contact Technical Service for further support.
Recommended products
Find a digital Reference Material for this product available on our online platform ChemisTwin® for NMR. You can use this digital equivalent on ChemisTwin® for your sample identity confirmation and compound quantification (with digital external standard). An NMR spectrum of this substance can be viewed and an online comparison against your sample can be performed with a few mouseclicks. Learn more here and start your free trial.
Legal Information
VETRANAL is a registered trademark of Merck KGaA, Darmstadt, Germany
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Sens. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
P Batjoens et al.
Journal of chromatography. A, 750(1-2), 127-132 (1996-10-25)
A more sensitive method was developed using the hyphenated technique of gas chromatography-mass spectrometry (GC-MS) supplementary to the official high-performance thin-layer chromatography (HPTLC) method. Even combined with less efficient extraction and clean-up methods, GC-MS is able to lower the detection
A M Attia et al.
Nucleosides & nucleotides, 18(10), 2307-2315 (2000-01-05)
N3-beta-D-glucopyranosyl, galactopyranosyl and xylopyranosyl 6-methyl-2-methylthiouracil and their 5-bromo derivatives have been synthesized by coupling an alpha-acetobromosugar with the corresponding thiouracil. The new modified thiouridine analogues were evaluated for their inhibitory activity against Human Immunodeficiency Virus (HIV) replication in MT-4 cells
D Matthias et al.
Atherosclerosis, 122(2), 201-216 (1996-05-01)
Following oral administration of methionine in high doses to normotensive (NR) and spontaneously hypertensive (SHR) rats, its degradation product, homocysteine (HC), which is markedly elevated in serum, exerts an angiotoxic action directed to the aorta. This is accompanied by considerable
T Merkulova et al.
American journal of physiology. Endocrinology and metabolism, 278(2), E330-E339 (2000-02-09)
During muscle development, an isozymic transition of the glycolytic enzyme enolase occurs from the embryonic and ubiquitous alphaalpha-isoform to the muscle-specific betabeta-isoform. Here, we demonstrate a stimulatory role of thyroid hormones on these two enolase genes during rat development in
Margarita S Chernov'yants et al.
Journal of pharmaceutical sciences, 99(3), 1567-1573 (2009-09-24)
The pre-equilibrium capillary zone electrophoretic (pre-eq CZE) method to determine association constants of active anionic forms of antithyroid drugs: 6-n-propyl-2-thiouracil (PTU), 6-methyl-2-thiouracil (MTU), 2-thiouracil (TU) with bovine serum albumin (BSA) under physiological pH 7.4 has been developed for the first
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service