All Photos(1)
About This Item
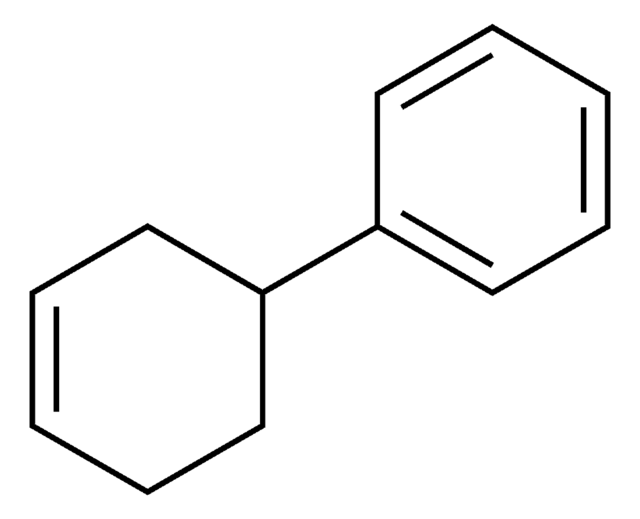
Linear Formula:
C6H5C6H9
CAS Number:
Molecular Weight:
158.24
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
95%
refractive index
n20/D 1.57 (lit.)
bp
251-253 °C (lit.)
mp
−11 °C (lit.)
density
0.994 g/mL at 25 °C (lit.)
SMILES string
C1CCC(=CC1)c2ccccc2
InChI
1S/C12H14/c1-3-7-11(8-4-1)12-9-5-2-6-10-12/h1,3-4,7-9H,2,5-6,10H2
InChI key
WCMSFBRREKZZFL-UHFFFAOYSA-N
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
217.4 °F - closed cup
Flash Point(C)
103.00 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
C E Cook et al.
Drug metabolism and disposition: the biological fate of chemicals, 12(2), 186-192 (1984-03-01)
In vitro metabolites of 1-phenylcyclohexene produced by the 10,000g supernatant fraction from rat liver homogenates were identified by a combination of spectrometric, chromatographic, and synthetic techniques. Initial oxidation occurred in the 3-position of 1-phenylcyclohexene to yield 1-phenyl-1-cyclohexen-3-one and 1-phenyl-1-cyclohexen-3-ol. Further
F C Law et al.
Drug and chemical toxicology, 7(3), 273-282 (1984-01-01)
The biliary excretion of 14C-phenylcyclohexene and its metabolites were studied in rats pretreated with an inducer or inhibitor of mixed-function oxidases or with an agent known to deplete liver glutathione. Pretreatment of rats with 3-methylcholanthrene or phenobarbital enhanced the biliary
A S Freeman et al.
Drug metabolism and disposition: the biological fate of chemicals, 10(6), 680-684 (1982-11-01)
Mice were exposed to the smoke from placebo marihuana cigarettes treated with phencyclidine hydrochloride (PCP . HCl). A dose-related decrement in motor performance was observed after exposure to the smoke from cigarettes containing 10-15 mg of PCP . HCl. Tissue
A K Chaturvedi et al.
Pharmacology, biochemistry, and behavior, 30(4), 1035-1043 (1988-08-01)
The interaction between phencyclidine (PCP) and its pyrolysis product, 1-phenylcyclohexene (PC), at metabolic level was evaluated in Swiss male mice (21-24 g). PC (1.1, 2.2 and 4.4 mmol/kg/day for 4 days, IP, in corn oil) treatment to mice induced the
D H Young et al.
Bioorganic & medicinal chemistry letters, 11(11), 1393-1396 (2001-05-30)
Phenylcyclohexenes (PCHs) [e.g., trans-4-nitro-5-(2,3,4-trimethoxyphenyl)cyclohexene, 2d] were found to bind weakly to the colchicine site of bovine tubulin, but are the first mimics of colchicine found to have high activity towards plant cells. Structure-activity relationships for PCHs and biphenyl AC-ring analogues
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service