69899
Monochlorobimane
suitable for fluorescence, ≥70.0% (HPCE)
Synonym(s):
mBCl, Chlorobimane
About This Item
Recommended Products
Quality Level
Assay
≥70.0% (HPCE)
form
powder
mp
135-136 °C (lit.)
solubility
DMF: soluble
DMSO: soluble
acetonitrile: soluble
methanol: soluble
fluorescence
λex 380 nm; λem 461 nm in methanol
λex 390 nm; λem 478 nm in 0.1 M phosphate pH 7.5 (after derivatization with glutathione)
suitability
suitable for fluorescence
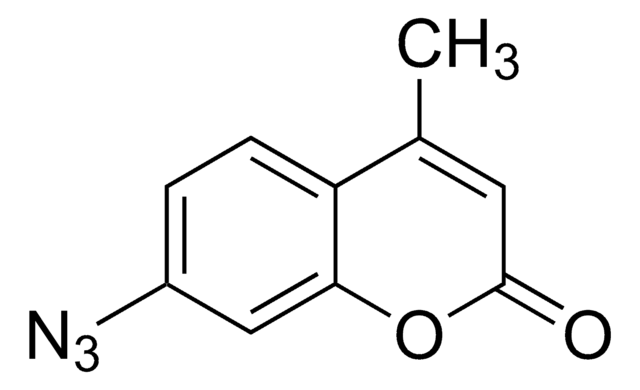
SMILES string
CC1=C(C)C(=O)N2N1C(CCl)=C(C)C2=O
InChI
1S/C10H11ClN2O2/c1-5-7(3)12-8(4-11)6(2)10(15)13(12)9(5)14/h4H2,1-3H3
InChI key
SUIPVTCEECPFIB-UHFFFAOYSA-N
General description
Monochlorobimane, also known as mBCl, is a non-fluorescent compound that forms a fluorescent complex upon reaction. The fluorescence is detected at 394/490nm.
Application
Packaging
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Fluorescence lifetime measurement is advantageous over intensity-based measurements. Applications include fluorescence lifetime assays, sensing and FLI.
Fluorescence lifetime measurement is advantageous over intensity-based measurements. Applications include fluorescence lifetime assays, sensing and FLI.
Fluorescence lifetime measurement is advantageous over intensity-based measurements. Applications include fluorescence lifetime assays, sensing and FLI.
Fluorescence lifetime measurement is advantageous over intensity-based measurements. Applications include fluorescence lifetime assays, sensing and FLI.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service