Purification using GST SpinTrap™
GST SpinTrap™ columns are designed for rapid, small-scale purification of GST-tagged proteins using mild conditions of affinity purification. More than 90% purity can be achieved in a single step. The columns are suitable for purification of multiple samples in parallel, for screening experiments, or for optimization of purification conditions.
Each microspin column contains 50 µl of Glutathione Sepharose 4B, enough to purify up to 500 µg of recombinant GST (rGST). The capacity varies with the nature of the GST-tagged protein and the binding conditions used. Refer to Appendix 5 (Characteristics of Glutathione Sepharose and HiTrap Benzamidine FF (high sub) media and columns) for the main characteristics of GST SpinTrap™.

Figure 3.6GST SpinTrap™ is a single-use column for rapid, small-scale purification of GST-tagged proteins.
Sample Preparation
For small-scale cultures, freeze/thaw or chemical lysis with commercial kits is recommended for cell lysis. Cytiva provides lysis kits for different expression systems: Mammalian Protein Extraction Buffer for mammalian expression systems and Yeast Protein Extraction Buffer Kit for yeast expression systems. For bacteria, several chemical lysis kits are available on the market.
Adjust the sample to the composition and pH of the binding buffer by additions from concentrated stock solutions; by diluting the sample with binding buffer; or by buffer exchange.
Pass the sample through a 0.22 µm or a 0.45 µm filter, and/or centrifuge it immediately before sample application. If the sample is too viscous, dilute it with binding buffer to prevent clogging; increase lysis treatment (sonication, homogenization); or add DNase/RNase to reduce the size of nucleic acid fragments. The binding properties of the target protein can be improved by performing a buffer exchange using a desalting column, for example, a PD SpinTrap™ G-25 column.
Note: Cell culture lysates may also be directly applied to the column without prior clarification.
Before starting the procedure, refer to page 32 for general considerations for purification of GST-tagged proteins.
The capacity of each SpinTrap™ column is 500 µg of GST-tagged protein. The following procedure is designed for lysates prepared from 2 to 12 ml of culture, which represents roughly 100 to 600 µl of lysate.
Perform purifications on GST SpinTrap™ using a standard microcentrifuge. Place the column in a 2 ml microcentrifuge tube to collect the liquid during centrifugation. Use a new 2 ml tube for every step (steps 1 to 5).
Recommended buffers can easily be prepared from the GST Buffer Kit.
Reagents Required |
|---|
Procedure
- Invert and shake the column repeatedly to resuspend the medium.
- Loosen the top cap one-quarter of a turn and twist/break off the bottom closure.
- Place the column in a 2 ml microcentrifuge tube and centrifuge for 30 s at 100 × g (approx. 1500 rpm in an Eppendorf™ 5415R, 24-position fixed-angle rotor) to remove the storage liquid.
- Remove and discard the top cap. Equilibrate the column by adding 600 µl of binding buffer. Centrifuge for 30 s at 100 × g.
- Add the sample (see Sample preparation). A suitable sample volume is 600 µl per column.
- Mix gently at room temperature for 5 to 10 min to ensure optimal binding of GST-tagged proteins to the Glutathione Sepharose 4B medium. Centrifuge for 30 s at 100 × g.
- Wash with 600 µl of binding buffer. Centrifuge for 30 s at 100 × g. Repeat the wash step once.
- Elute the target protein twice with 200 µl of elution buffer. Centrifuge for 30 s at 100 × g, and collect the purified sample. The first 200 µl will contain the majority of the target protein.
Yields of tagged protein can be increased by repeating the elution step two or three times and pooling the eluates.
It is possible to make several sample applications as long as the binding capacity of the column is not exceeded.
Application example
Rapid screening of GST-tagged proteins using GST SpinTrap™
E. coli transformants containing cDNA expressing a GST-tagged human myoglobin were randomly selected, expressed, and purified using GST SpinTrap™ columns. A human myoglobin cDNA was ligated to linearized pGEX-5X-1 and used to transform E. coli BL21 cells. Twenty-four randomly selected colonies were used to inoculate 3 ml cultures, which were grown overnight. Expression was induced with IPTG for 2 h. Lysates were prepared from 1.5 ml aliquots of each culture by a freeze-thaw procedure and applied to GST SpinTrap™ columns. Aliquots of each reduced glutathione eluate were applied to an SDS gel for analysis by SDS-PAGE (Figure 3.7). The results showed that 7 of the 24 transformants expressed the GST- tagged myoglobin.
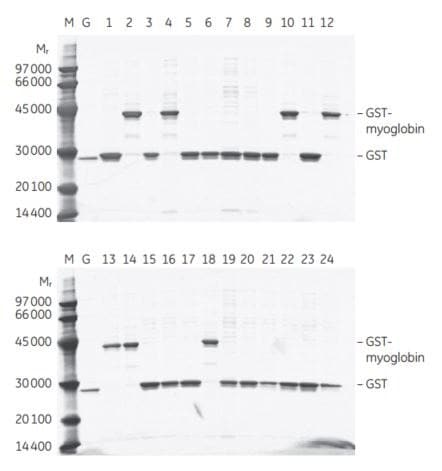
Figure 3.7SDS-PAGE analysis of eluates from a screening of 24 randomly selected E. coli transformants containing cDNA expressing GST-tagged human myoglobin. M = LMW-SDS Marker Kit. G = purified rGST. Lanes 1 to 24 contain products eluted from the GST SpinTrap™ columns using reduced glutathione.
Pour continuer à lire, veuillez vous connecter à votre compte ou en créer un.
Vous n'avez pas de compte ?