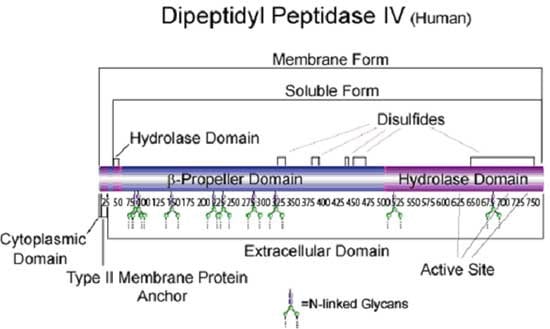
Dipeptidyl Peptidase IV
Enzyme Commission (EC) Number: 3.4.14.5
Primary Accession Number: P27487 (DPP4_HUMAN)
Synonyms:
DPPIV, dipeptidyl aminopeptidase IV, CD26, glycoprotein GP110, glycylproline aminopeptidase, T cell triggering molecule Tp103, X-PDAP, THAM, and adenosine deaminase binding protein (ADAbp).
Physical Properties and In Vivo Processing
Native DPPIV is a ubiquitous type II transmembrane glycoprotein and a serine protease of the S9 prolyl-oligopeptidase family. In vivo, it is synthesized with a signal peptide which functions as the membrane anchoring domain.1,2 There is an 88% sequence homology between the human and porcine kidney enzymes. 3 Both the human and porcine kidney enzymes exist as homodimers with a subunit molecular weight of approx. 30 kDa. The high mannose 100 kDa DPPIV precursor is processed in the Golgi to yield a 124 kDa heavily N-and O-linked mature glycoprotein.4 It is then sorted to the apical membrane through the concerted action of both N- and O-linked glycans and it’s association with lipid microdomains.5 The porcine enzyme contains 18.3% carbohydrates, of which the glycan composition is 0.9% fucose, 3.4% mannose, 5.1% galactose, 8.2% glucosamine, and 0.7% sialic acid.1,2
pI: 5.26 (porcine)
Extinction Coefficient: E1% = 11.5 (280 nm)7 (porcine)
Physiological Properties and Clinical Implications
DPPIV is highly expressed on endothelial cells, epithelial cells and lymphocytes.8,9,10 It is also present in plasma in its soluble form.11
DPPIV is involved in the regulation of several important physiological processes: 12,13,14
- Immune system
- Inflamation
- CNS
- Endocrine functions
- Bone marrow mobilization
- Cancer growth
- Cell adhesion
- Glucose hemostasis
- Sepsis/severe infection
Natural DPPIV substrates include: 12,13
- Glucagon-like peptides-1 & 2
- Glucose–dependent insulinotropic peptide
- Neuropeptide Y
- Substance P
- Peptide YY
- IGF-1
- Prolactin
- hCGα
- Growth Hormone Releasing Factor
- LHα
- Thyrotropinα
- Enkephalins
- Vasostatin
- Eotaxin
- Interferon-γ inducible protein
- IFN-inducible T-cell alpha-chemoattractant
- Procalcitonin15
- Macrophage-derived chemokine
- Monokine induced by Interferon-γ
Natural DPPIV ligands include:12,16,17,18,19
- Adenosine deaminase-I and II
- Renal Na+/H+ ion exchanger NHE3 isoform
- Fibronectin
DPPIV inhibitors have been found to improve glucose tolerance and preserve islet function in mice.20 DPPIV inhibitors were found to block entry of HIV-1 or HIV-2 into T lymphoblastoid and monocytoid cell lines.21
Specificity and Kinetics
DPPIV has a post-proline dipeptidyl aminopeptidase activity that hydrolyzes N-terminal dipeptides from the unsubstituted N-terminus of peptides with the sequence of X-Pro-Z and X-Ala-Z.
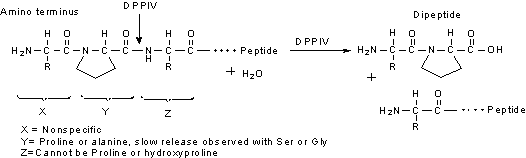
The optimum pH for activity is 7.4-8.7.13,22 At pH 7.0 DPPIV exhibits about 45% of maximal activity and at pH 9.6 it exhibits about 90% of maximal activity. Below pH 5.0 the enzyme is essentially inactive.11,23
We determine the enzymatic activity of DPPIV using the chromogenic substrate Gly-Pro-p-nitroanilde. Reported KM values are 0.66 mM for Gly-Pro-2-naphthylamide24 and 1 mM for Ala-Ala-2-naphthylamide.23
Enzymatic Assay
Unit Definition: One unit will produce 1.0 µmole of p-nitroaniline from Gly-L-Pro p-nitroanilide per min in 100 mM Tris-HCl at pH 8.0 at 37 °C.
Stopped Rate Spectrophotometric Determination using a Microplate Reader
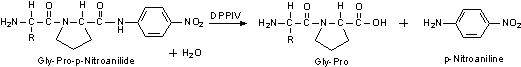
CONDITIONS: T = 37 °C, pH = 8.0, A405nm, Inc. = 15 minutes
REAGENTS:
- 0.1 M Tris, pH 8.0 at 37 °C
- 1 mM Gly-Pro-pNA Solution
- 1 mM p-Nitroaniline Solution (pNA)
- Dipeptidyl Peptidase Enzyme Solution
(Immediately before use prepare the following in cold reagent A. Dilute to obtain 0.04 - 0.08 units/mL.)
PROCEDURE:
- Set up microtitre 96-well plate with the following standards:
S1 = 20 µL 1 mM pNA (20 nmols)
S2 = 40 µL 1 mM pNA (40 nmols)
S3 = 60 µL 1 mM pNA (60 nmols)
S4 = 80 µL 1 mM pNA (80 nmols)
S5 = 100 µL 1 mM pNA (100 nmols)
Make all wells up to 0.1 mL with Reagent A. - Pipette in enzyme samples as follows:
T1 = 10 µL
T2 = 20 µL
T3 = 30 µL
T4 = 40 µL
T5 = 50 µL
Make all wells up to 0.1 mL with Reagent A. - Add 0.1 mL of Reagent B to each well (including standards) to start the reaction.
- Incubate at 37 °C for 15 minutes.
- Read absorbance at 405 nm in a microplate reader.
CALCULATIONS:
Plot a standard curve of A405nm versus nmoles of pNA. Calculate nmoles/minute and hence from this determine mmoles/min/mL of enzyme.
FINAL ASSAY CONCENTRATION:
In a 0.2 mL reaction mix, the final concentrations are 100 mM Tris, 0.5 mM Gly-Pro-pNA, and varying amounts of enzyme.
This procedure is for informational purposes. For a current copy of our quality control procedure, please contact our Technical Service Department.
Synthetic Substrates
Gly-Pro 4-methoxy-β-naphthylamide
Gly-Pro 4-methoxy-β-naphthylamide
Gly-Pro p-nitroanilide hydrochloride
Gly-Pro p-nitroanilide p-toluenesulfonate salt
Gly-Pro-7-amido-4-methylcoumarin hydrobromide
Inhibitors
diisopropyl fluorophosphate
phenylmethanesulfonyl fluoride
Ile-Pro-Ile (Diprotin A)
KR-62436
Enzymes
Dipeptidyl Peptidase, human
Dipeptidyl Peptidase from porcine kidney
Solubility and Stability
The porcine enzyme (Prod. No. D7052) is supplied as a lyophilized powder and is soluble in 100 mM Tris HCl buffer, pH 8.0 (1 vial/mL), yielding a clear, colorless solution. The human enzyme (Prod. No. D4943) is supplied as a solution in 10 mM Tris-HCl pH 7.6, 200 mM NaCl, 1 mM EDTA, 10 % glycerol.
As supplied, these products should be stable for a minimum of one year when stored properly at –20 °C.
References
Pour continuer à lire, veuillez vous connecter à votre compte ou en créer un.
Vous n'avez pas de compte ?