Reproducible Scaling of a Cell Retention Filter Using the Cellicon® Cell Retention Solution
The Role of Cell Retention in Intensified Upstream Workflows
Monoclonal antibody production processes continue to evolve and intensify. One strategy for intensifying the upstream workflow is the use of perfusion which can support higher cell densities and longer process durations, leading to greater utilization of manufacturing facility assets. Perfusion also allows for continuous processing and offers key advantages over batch and fed-batch processes such as greater cell-mass production and manufacturing flexibility, without the need for larger bioreactors.
At the core of perfusion technology is the cell retention device which enables removal of spent medium from the cell culture while retaining high numbers of viable cells within the bioreactor vessel. Advances in cell retention technologies are essential for the continued evolution of perfusion manufacturing processes for monoclonal antibodies and recombinant proteins.
Design and scalability of the cell retention device, the recirculation pump type and flow rate, and the rate of media turnover all affect the performance of the upstream workflow. As such, it is important to select a cell retention solution that can scale over a wide range of bioreactor volumes to enable predictable results.
The Cellicon® Cell Retention Solution consists of the Cellicon® Filter Assembly, Mobius® Cell Retention System, and Bio4C ACE™ software. To address the complexities of scaling, the Cellicon® Filter Assembly was designed with an emphasis on providing reproducible performance across scales.
This page describes the design attributes of the Cellicon® Filter Assembly and how they simplify process scale-up.
Cellicon® Filter Assembly Design Enables Scalability
The Cellicon® Filter Assembly was designed to ensure linear scalability from lab-scale through to process scale. Key design elements and process parameters include device dimensions, membrane area, crossflow rate, and perfusion rate.
Dimensions
The Cellicon® filter assembly uses a flat sheet format to optimize scalability. The channel height and length remain consistent across all sizes, while the channel width expands from lab to process scale. Augmentation of the membrane area across process scale devices is achieved by increasing the number of feed channels. With the dimensions of each channel held constant, fluid flow velocity over the membrane surface remains the same, as crossflow rate and the number of channels are increased by scale. Using this filter design strategy, performance at larger scales can be reliably predicted by performance at lab scale.
Membrane Area
The membrane area for each filter was chosen to sustain a consistent ratio of bioreactor working volume to membrane area, ensuring reliable performance from one size to the next. Table 1 shows that there is a consistent ratio of 236-265 liters of bioreactor working volume per square meter of membrane area across all scales.
The ratio of membrane area to bioreactor volume should be considered when designing scaling experiments. For example, when designing a lab-scale process to mirror a production process with a 1,500 L working volume and a 7.6 m² filter, a volume to area ratio of 197 L/m² would be targeted. Therefore, a scaled-down process using the 0.01 m² filter could be designed at 1.97 L (197 L/m² * 0.01 m²). Under these conditions, lab-scale results should predict production-scale performance, provided all other scalability factors are also kept constant.
Crossflow/Shear Rate
The target crossflow rate of each filter size was chosen to sustain a wall shear at the membrane surface of 2,500 s-1. By maintaining a constant shear rate across scales, uniform sweeping of the membrane surface is assured, minimizing surface fouling of the membrane, and delivering predictable results upon scale-up. The recommended crossflow rates shown in Table 2 serve as an initial guide and can be adjusted as needed to modify the shear on the membrane surface.
All feed pumps rely on the same centrifugal technology to provide uniform flow through the fluid path. The pump sizes for each scale were chosen to achieve the target flow rates while minimizing pump shear by keeping speeds within the lower range of the pump's capacity.
Filter throughput can be improved by raising the crossflow rate, which increases the sweeping at the surface of the membrane to prevent fouling (Figure 1). When the crossflow rate is increased, additional shear can be imparted on the cells due to higher pump speeds. Therefore, process development is recommended to ensure operation within the safe range for cell health while achieving filter longevity targets.
Effect of Crossflow on Performance
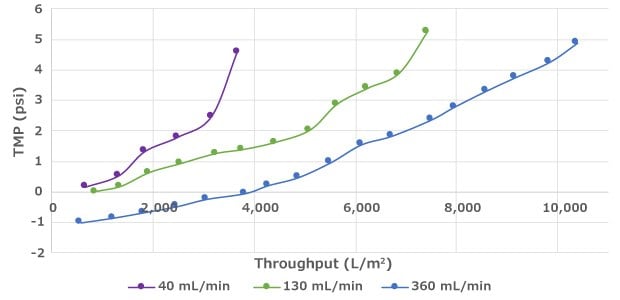
Figure 1.Increased crossflow rate can lead to improved filter throughput prior to fouling.
Perfusion Rate
The Cellicon® Filter is designed to support a broad range of fluxes to achieve the variable perfusion rates needed for different cell lines and processes. Since the membrane area is scaled linearly with bioreactor volume, flux can also be scaled linearly. The perfusate flux should be maintained at less than 22 L/m²*hr, or LMH; this yields a maximum filtration rate of about 2 VVD for the peak working volume at each scale, as depicted in Table 3.
Steady state perfusion runs at 60E6 cells/mL were conducted to evaluate the impact of flux on filter throughput (Figure 2). At the evaluated cell densities, filter throughput was not impacted over the range of fluxes evaluated (13 and 26 LMH). However, filter throughput may decrease if the flux is above a critical value, which will vary by cell line and bioreactor process conditions. Therefore, flux is an important parameter to evaluate during scale-down studies.
Impact of Flux on Performance
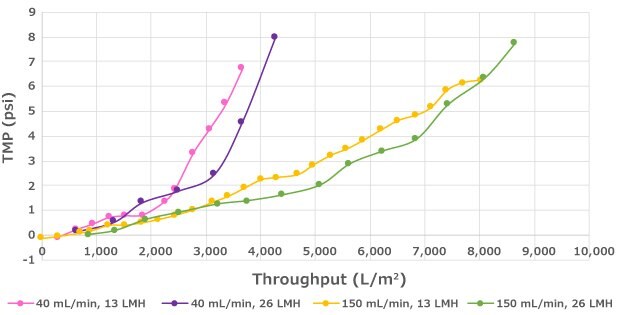
Figure 2.Decreased flux can lead to longer run duration but has no significant impact on throughput within the flux range that was evaluated.
Demonstration of Scalability
To evaluate scalability, a comparison of a lab-scale Cellicon® filter running in parallel with either 50 L or a 200 L process-scale Cellicon® filter was performed using the same cell culture (Figure 3). In each experiment, cells were maintained in an exponential growth phase by gradually increasing the perfusion rate daily to support the cell density in each bioreactor. By adhering to the scaling parameters of flux and crossflow rate within each filter size, and exposing both to the same cell culture process, similar performance between lab-scale and process scale filters was observed. These results confirm that the filter design has been optimized to enable linear scalability in terms of fouling rate.
Cellicon® Filter Scalability

Figure 3.To evaluate scalability, process scale Cellicon® filters were run in parallel with lab-scale Cellicon® filters, results show matching fouling rates across filter sizes.
Scalability of Cell Retention for Intensified Upstream Processes
Implementation of the Cellicon® Filter Assembly removes uncertainty when it comes to scaling perfusion cell culture processes. Designed for scalability, the Cellicon® Cell Retention Solution safeguards filter performance from the start, ensuring process longevity and productivity at all stages.
The filter assembly uses a flat sheet format for optimized linear scalability, maintaining uniformity across scales through constant channel dimensions and pump types. By maintaining a steady bioreactor volume to membrane area ratio and appropriately scaling crossflow and perfusion rates, reproducible performance can be anticipated across different scales. For instance, the results of a lab-scale process using a 0.01 m2 device filter are predictive of performance at a 2,000 L production scale. This allows for accelerating process development and progress of a biopharmaceutical campaign to manufacturing.
Pour continuer à lire, veuillez vous connecter à votre compte ou en créer un.
Vous n'avez pas de compte ?