Chiral Diols
Apart from numerous examples using TADDOLs in metal-catalyzed asymmetric reactions, Rawal recently reported that TADDOLs could be used as Brønsted acid organocatalysts in highly stereoselective hetero-Diels–Alder reactions.1 The reaction of an electron-rich diene with benzaldehyde using 10 mol % TADDOL (395242) provides the dihydropyrone as a single stereoisomer (Scheme 1).
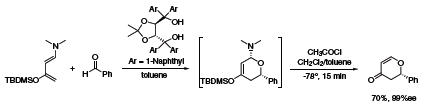
Scheme 1.
The α-amination of carbonyl compounds has also been accomplished by using the 1-naphthyl TADDOL derivative as a Brønsted acid catalyst (Scheme 2).2 The reaction of different enamines with nitrosobenzene gave exclusively the N-regioisomers in a highly enantioselective manner.
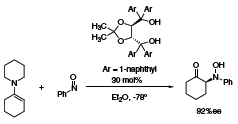
Scheme 2.
A highly enantioselective addition of cyclohexenone to different aldehydes (asymmetric Morita–Baylis–Hillman reaction) catalyzed by octahydro-BINOL-derived Brønsted acid (669172) was reported by Schaus (Scheme 3).3 Important for achieving high enantioselectivity were both the partial saturation and substitution at the 3,3’-positions of the BINOL derivative.
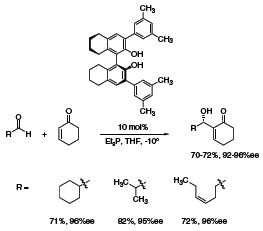
Scheme 3.
Pour continuer à lire, veuillez vous connecter à votre compte ou en créer un.
Vous n'avez pas de compte ?