Buchwald Phosphine Ligands for Cross Coupling
The impact of cross-coupling methodologies to form C-C bonds is paramount in organic synthesis.1 Of these, Suzuki-Miyaura coupling is among the most powerful transformations available as it has broad scope and wide functional group tolerance.2 To this end, notable advances have been made in the laboratories of Prof. Stephen Buchwald at MIT.
We offer a series of Buchwald Ligands successfully used in processes, including Suzuki-Miyaura coupling, amination, amidation, enolate arylation, Sonogashira coupling and C-O bond formation (Table 1). While each ligand has documented utility, application of dimethoxy-substituted ligand 1, S-Phos, leads to a Pd-catalyst system with unprecedented scope, reactivity, and stability for Suzuki-Miyaura coupling processes.3 Selected examples (Table 2) illustrate the success of this system with respect to aryl chloride substrates, the generation of truly hindered biaryls, and heteroaryl cross-couplings.
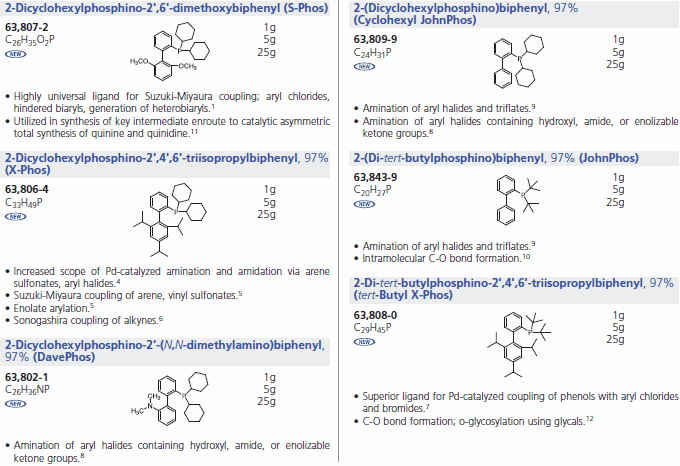
Buchwald Phosphine Ligands (Table 1)
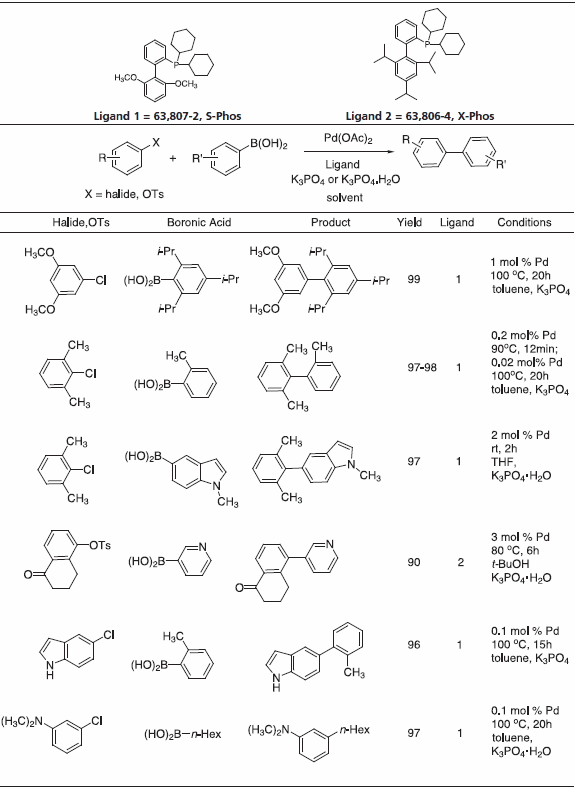
Table 2.
Triisopropyl-substituted ligand 2, X-Phos has key applications to Pd-catalyzed C-N bond formation.4 Table 3 gives examples that typify the expanded scope of this process utilizing X-Phos. X-Phos has also been successfully applied to Suzuki-Miyaura couplings with arene and vinyl sulfonates5 (Table 2), as well as Sonogashira coupling of alkynes6 (Table 4). 2-Di-t-butylphoshino-2’,4’,6’- triisopropyl ligand has been found to be a superior ligand for Pd-catalyzed coupling of phenols with aryl bromides and chlorides.7
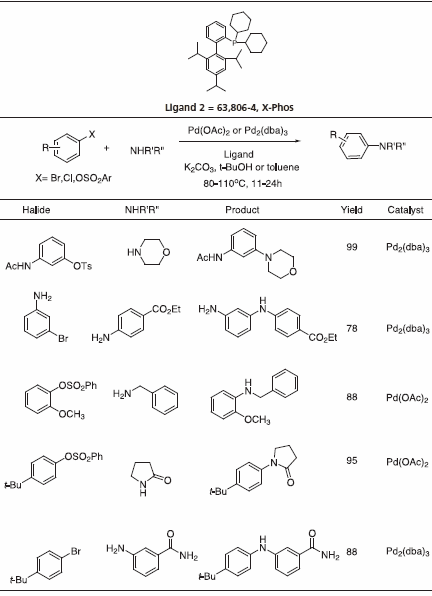
Table 3.
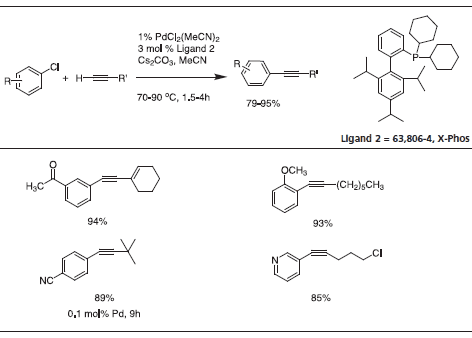
Table 4.
References
Pour continuer à lire, veuillez vous connecter à votre compte ou en créer un.
Vous n'avez pas de compte ?