234923
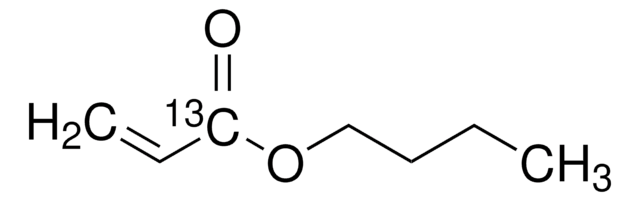
Butyl acrylate
≥99%, contains 10-60 ppm monomethyl ether hydroquinone as inhibitor
Synonyme(s) :
n-Butyl acrylate
About This Item
Produits recommandés
Densité de vapeur
>1 (vs air)
Niveau de qualité
Pression de vapeur
3.3 mmHg ( 20 °C)
Pureté
≥99%
Forme
liquid
Température d'inflammation spontanée
559 °F
Contient
10-60 ppm monomethyl ether hydroquinone as inhibitor
Limite d'explosivité
9.9 %
Indice de réfraction
n20/D 1.418 (lit.)
Point d'ébullition
145 °C (lit.)
Densité
0.894 g/mL at 25 °C (lit.)
Chaîne SMILES
CCCCOC(=O)C=C
InChI
1S/C7H12O2/c1-3-5-6-9-7(8)4-2/h4H,2-3,5-6H2,1H3
Clé InChI
CQEYYJKEWSMYFG-UHFFFAOYSA-N
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Catégories apparentées
Description générale
Butyl acrylate undergoes radical copolymerization with benzoxazine containing a vinyl group to afford copolymers. Heck coupling reactions of aryl bromides with n-butyl acrylate mediated by phosphine-imidazolium salt have been reported. Copolymerization of styrene and n-butyl acrylate by ATRP catalyzed by CuBr/4,4′-di(5-nonyl)-2,2′-bipyridine has been described.
Application
- An electrolyte additive in lithium-ion batteries to improve their low-temperature performance. The addition of BA to the electrolyte led to a significant improvement in the low-temperature performance of the battery, including enhanced ionic conductivity and improved rate capability.
- A monomer to synthesize a shape memory polymer network that contains magnetic nanoparticles for various applications, including actuators and biomedical devices.
- A monomer for the preparation of a polymeric semiconductor with intrinsically stretchable properties. This polymer material is used as a component in field-effect transistor applications.
- Poly(butyl acrylate) particles.
- Poly(butyl acrylate-b-acrylic acid) block copolymer.
- Amphiphilic charged diblock copolymers poly(butyl acrylate)-b-poly(acrylic acid).
- Poly(n-butyl acrylate), via atom transfer radical polymerization (ATRP) of n-butyl acrylate in the presence of CuIBr/4,4′-di(5-nonyl)-2,2′-bipyridine (catalyst).
Mention d'avertissement
Warning
Mentions de danger
Classification des risques
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Aquatic Chronic 3 - Eye Irrit. 2 - Flam. Liq. 3 - Skin Irrit. 2 - Skin Sens. 1 - STOT SE 3
Organes cibles
Respiratory system
Code de la classe de stockage
3 - Flammable liquids
Classe de danger pour l'eau (WGK)
WGK 1
Point d'éclair (°F)
98.6 °F - closed cup
Point d'éclair (°C)
37 °C - closed cup
Équipement de protection individuelle
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Articles
Monomers for ophthalmic use aim for purity, reliability, and comfort, driving innovation for affordable contact lenses.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique