Catalysts for Carbonylation
Professor Geoffrey Coates and co-workers at Cornell University have reported the preparation and use of catalysts composed of an oxophilic Lewis acid and a cobalt tetracarbonyl anion (1–3, Figure 1) for the ring expansive carbonylation of epoxides to b-lactones and b-lactones to succinic anhydrides (Scheme 1).1,2 Catalyst 3 provides access to succinic anhydrides directly from epoxides via a one-pot double carbonylation.3 The catalysts are prepared from readily available ligand metal chlorides ([LnM]Cl) and NaCo(CO)4.1,2
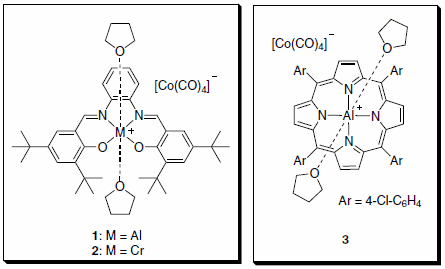
Figure 1.
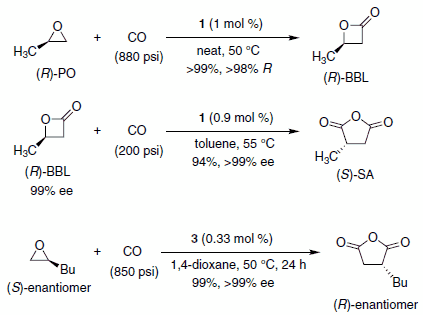
Scheme 1.
A generalized catalytic cycle for b-lactone formation (Figure 2) involves initial replacement of a THF ligand at the metal center by the epoxide oxygen followed by nucleophilic ring opening at the less substituted carbon by the cobalt tetracarbonyl anion. Insertion of CO then proceeds rapidly followed by ring closure and regeneration of the catalyst.4 Consistent with this mechanism is the observed regioselectivity, retention of stereochemistry at the substituted epoxide carbon and inversion of stereochemistry at the b-carbon of the lactone (Figure 2, path A). A side reaction, observed at low CO pressure, is ketone formation resulting from elimination of HCo(CO)4 (path B). The ring expansion of b-lactone to succinic anhydride occurs via a similar catalytic cycle. A more detailed discussion of the two catalytic mechanisms has been published.3,5
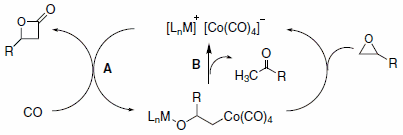
Figure 2.
These single-stage carbonylations proceed quickly in the absence of solvent or in polar, non-donating solvents such as ethers (e.g., DME). Catalyst loadings are typically 0.5–2 mol %. The reactions provide high yields of essentially pure products under CO pressures of 200 psi or higher. Use of catalyst 2 enables the ring expansions to be run at lower CO pressures, even as low as one atmosphere.6 However, at 1 atm CO, small amounts of ketone are formed (path B). The reactions are tolerant of functionality in the alkyl substituent such as silyl ethers, olefins, and esters (Table 1 and Table 2).2,6
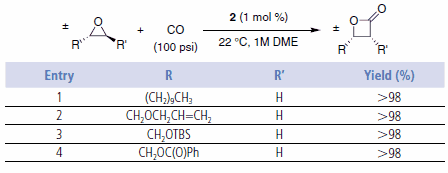
Table 1.
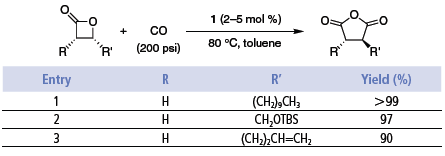
Table 2.
The double carbonylation process catalyzed by 3 proceeds in stages with b-lactone carbonylation occurring only on completion of the epoxide carbonylation. The solvent of choice is 1,4-dioxane. The reactions proceed with CO pressures as low as 100 psi. However, for the purposes of this study, the carbonylations were run at 850 psi. At lower pressures, or in the absence of CO, the epoxide rearranges to ketone. Without solvent small amounts of polyesters are formed. The double carbonylation process provides high yields of products requiring little, if any, purification and is tolerant of functional groups such as alkyl, aryl, olefins, ethers, silyl ethers, esters, amides, and nitriles (Table 3).3
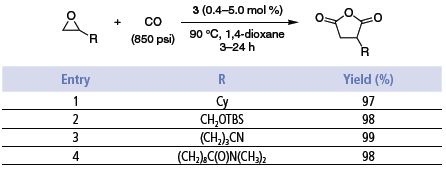
Table 3.
Oxetane2 and N-tosyl aziridine 47 have also been reported to undergo carbonylative ring expansion (Scheme 2).
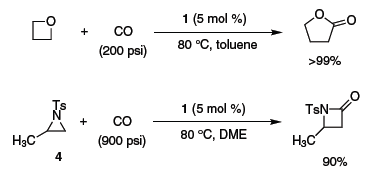
Scheme 2.
References
To continue reading please sign in or create an account.
Don't Have An Account?