UHPLC/MS/MS Analysis of 25 Small Molecule Pharmaceutical Compounds on Ascentis® Express RP-Amide
Materials
analytical column
mobile phase component
standard
Atrazine
certified reference material, TraceCERT®, Manufactured by: Sigma-Aldrich Production GmbH, SwitzerlandBenzoylecgonine solution
1.0 mg/mL in methanol, ampule of 1 mL, certified reference material, Cerilliant®Amitriptyline hydrochloride solution
1.0 mg/mL in methanol, ampule of 1 mL, certified reference material, Cerilliant®Haloperidol solution
1.0 mg/mL in methanol, ampule of 1 mL, certified reference material, Cerilliant®Imipramine hydrochloride solution
1.0 mg/mL in methanol (as free base), ampule of 1 mL, certified reference material, Cerilliant®Mianserin hydrochloride solution
1.0 mg/mL in methanol (as free base), ampule of 1 mL, certified reference material, Cerilliant®CONDITIONS
column
Ascentis Express RP-Amide, 10 cm x 2.1 mm I.D., 2.7 μm particles (53913-U)
mobile phase
[A] 0.1% acetic acid, 5 mM ammonium acetate in water; [B] 5 mM ammonium acetate in acetonitrile
gradient
5% B for 1 min; to 43% B in 6 min; held for 1 min; to 80% B in 4 min; held for 1 min; to 5% B in 1 min; held for 3 min
flow rate
0.4 mL/min
detector
MS, ESI(+) MRM
injection
1 μL
Description
Application
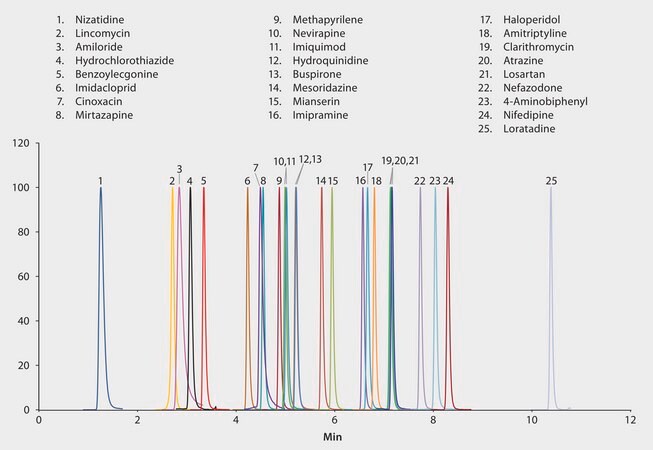
This application highlights the advantage of an embedded polar group (EPG) HPLC phase for the separation of various small molecules. The use of an Ascentis® RP-Amide column allowed for the development of a single UPLC-MS/MS method for the analysis of 25 pharmaceuticals that varied significantly in both chemical and physical properties (log P, pKa, etc.). The presence of an embedded amide group allowed for retention of more polar analytes in comparison to a traditional C18 phase.
Legal Information
Ascentis is a registered trademark of Merck KGaA, Darmstadt, Germany