921947
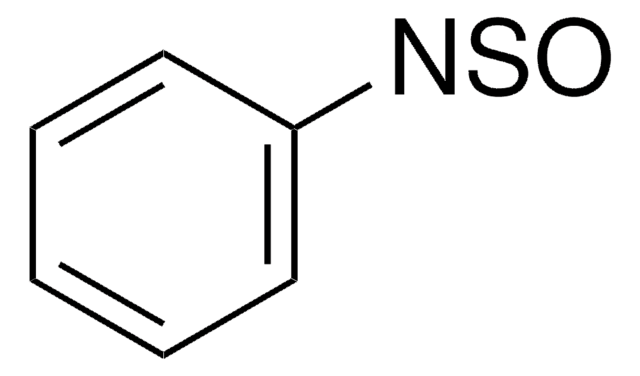
N-Sulfinyl-O-(tert-butyl)hydroxylamine
≥95%
Synonym(s):
t-BuONSO
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C4H9NO2S
CAS Number:
Molecular Weight:
135.18
MDL number:
UNSPSC Code:
12352116
Recommended Products
Application
t-BuONSO is a stable reagent active for the synthesis of primary sulfonamides when used in conjunction with organolithium or Grignard reagents. t-BuONSO is tolerant of a variety of alkyl and aryl substrates and can be used in the synthesis of many medicinally relevant compounds.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Resp. Sens. 1 - Self-react. C - Skin Sens. 1
Storage Class Code
5.2 - Organic peroxides and self-reacting hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Thomas Q Davies et al.
Journal of the American Chemical Society, 142(36), 15445-15453 (2020-08-26)
Sulfoximines and sulfonimidamides are promising compounds for medicinal and agrochemistry. As monoaza analogues of sulfones and sulfonamides, respectively, they combine good physicochemical properties, high stability, and the ability to build complexity from a three-dimensional core. However, a lack of quick
Ze-Xin Zhang et al.
Journal of the American Chemical Society, 141(33), 13022-13027 (2019-08-10)
Sulfondiimines-the double aza-analogues of sulfones-hold significant potential as leads in discovery chemistry, yet their application in this arena has been held back by the scarcity of appropriate synthetic routes. Existing methods employ sulfides as substrates, and rely on consecutive imination
Thomas Q Davies et al.
Organic letters, 22(24), 9495-9499 (2020-11-26)
Sulfonamides have played a defining role in the history of drug development and continue to be prevalent today. In particular, primary sulfonamides are common in marketed drugs. Here we describe the direct synthesis of these valuable compounds from organometallic reagents
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service



![(Ir[dF(CF3)ppy]2(dtbpy))PF6](/deepweb/assets/sigmaaldrich/product/structures/982/913/02dd8ddd-6deb-40a0-ab9b-07b18f1abb09/640/02dd8ddd-6deb-40a0-ab9b-07b18f1abb09.png)


![1,4-Diazabicyclo[2.2.2]octane bis(sulfur dioxide) adduct ≥95% (sulfur, elemental analysis)](/deepweb/assets/sigmaaldrich/product/structures/158/739/a9df497b-883d-40f1-ac45-bf699dcee9f9/640/a9df497b-883d-40f1-ac45-bf699dcee9f9.png)


