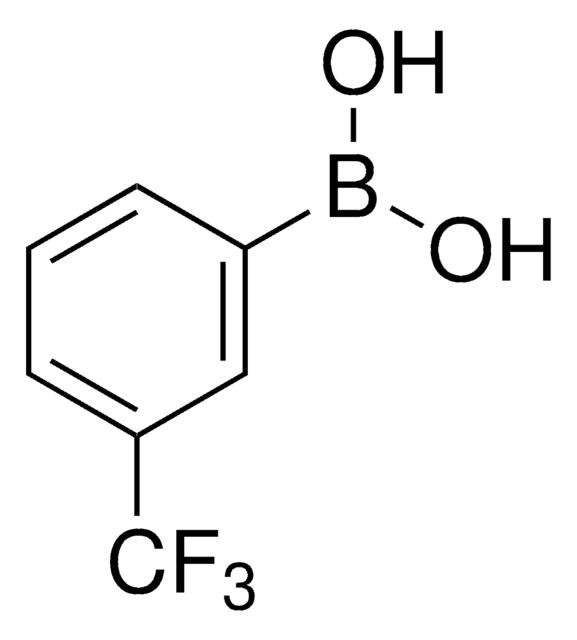
417548
4-Chlorophenylboronic acid
95%
Synonym(s):
(p-Chlorophenyl)boronic acid, 4-Chlorobenzeneboronic acid, NSC 25408, p-Chlorobenzeneboronic acid
About This Item
Recommended Products
Quality Level
Assay
95%
mp
284-289 °C (lit.)
functional group
chloro
SMILES string
OB(O)c1ccc(Cl)cc1
InChI
1S/C6H6BClO2/c8-6-3-1-5(2-4-6)7(9)10/h1-4,9-10H
InChI key
CAYQIZIAYYNFCS-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
- Palladium-catalyzed direct arylation.[1]
- Cyclopalladation.[2]
- Tandem-type Pd(II)-catalyzed oxidative Heck reaction and intramolecular C-H amidation.[3]
- Copper-mediated ligandless aerobic fluoroalkylation.[4]
- Pd-catalyzed arylative cyclization.[5]
- Ruthenium catalyzed direct arylation.[6]
- Ligand-free copper-catalyzed coupling reactions.[7]
- Regioselective arylation and alkynylation by Suzuki-Miyaura and Sonogashira cross-coupling reactions.[8]
It can also be used to prepare:
- Substituted diarylmethylidenefluorenes via Suzuki coupling reaction.[9]
- Baclofen lactam by Suzuki coupling of a pyrrolinyl tosylate, followed by hydrogenation reaction.[10]
- Palladium(II) thiocarboxamide complexes as Suzuki coupling catalysts.[11]
- Biaryls by Suzuki reactions of aryl chlorides, bromides, and iodides with arylboronic acids.[12]
Other Notes
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Active Filters
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service