49866
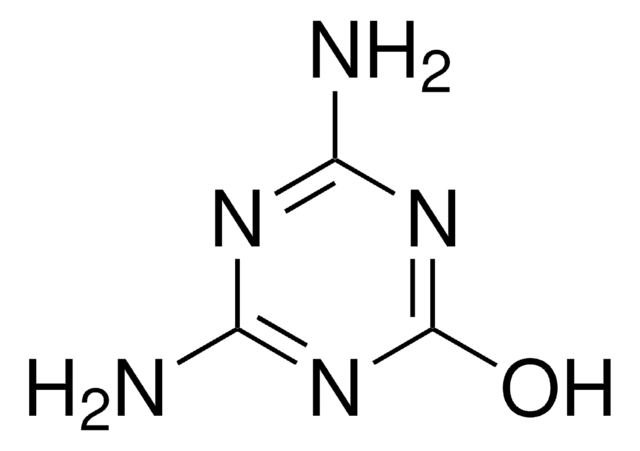
Ammelide
analytical standard
Synonym(s):
2-Amino-1,3,5-triazine-4,6-dione
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C3H4N4O2
CAS Number:
Molecular Weight:
128.09
EC Number:
MDL number:
UNSPSC Code:
85151701
PubChem Substance ID:
NACRES:
NA.24
Recommended Products
grade
analytical standard
Quality Level
Assay
≥98.0% (GC)
shelf life
limited shelf life, expiry date on the label
technique(s)
HPLC: suitable
gas chromatography (GC): suitable
application(s)
cleaning products
cosmetics
food and beverages
personal care
format
neat
SMILES string
Nc1nc(O)nc(O)n1
InChI
1S/C3H4N4O2/c4-1-5-2(8)7-3(9)6-1/h(H4,4,5,6,7,8,9)
InChI key
YSKUZVBSHIWEFK-UHFFFAOYSA-N
General description
Ammelide (AMD), a 1,3,5-triazine compound, is one of the hydrolysis by-products of melamine. It is most commonly found as an impurity in melamine and its structural analog, cyanuric acid feedstocks.
Ammelide can be synthesized via hydrolysis of 2-amino-4,6-dichloro-s-triazine in the presence of acid or base or by the reaction between urea and cyanogen iodide at 130-140 degrees.
Application
Ammelide may be used as an analytical reference standard for the determination of the analyte in milk, milk products and infant formula using chromatography techniques.
Refer to the product′s Certificate of Analysis for more information on a suitable instrument technique. Contact Technical Service for further support.
Packaging
Bottomless glass bottle. Contents are inside inserted fused cone.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Rapoport, L. and Smolin, ME
The Chemistry of Heterocyclic Compounds, 26 (2009)
R W Eaton et al.
Journal of bacteriology, 173(3), 1363-1366 (1991-02-01)
DNA encoding the catabolism of the s-triazines ammelide and cyanuric acid was cloned from Pseudomonas sp. strain NRRLB-12228 and Klebsiella pneumoniae 99 with, as a probe, a 4.6-kb PstI fragment from a third strain, Pseudomonas sp. strain NRRLB-12227, which also
Paul M Murphy et al.
Proceedings of the National Academy of Sciences of the United States of America, 106(23), 9215-9220 (2009-05-28)
Altering the specificity of an enzyme requires precise positioning of side-chain functional groups that interact with the modified groups of the new substrate. This requires not only sequence changes that introduce the new functional groups but also sequence changes that
Limin He et al.
Journal of separation science, 32(19), 3310-3318 (2009-09-02)
A method for molecularly imprinted SPE of the melamine from environmental water samples was investigated. Cyromazine-imprinted polymers were synthesized in water-methanol systems for the selective extraction of melamine from aqueous samples, followed by HPLC analysis. Molecular recognition properties and binding
Mechanistic and spectral investigation on the deamination of ammeline and ammelide
Almatarneh HM, et al.
Computational & Theoretical Chemistry, 1117, 92-99 (2017)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service