MAB1913-C
Anti-Procollagen Type I Antibody, CT, clone PCIDG10 (Ascites Free)
clone PCIDG10, from mouse
Synonym(s):
Collagen alpha-1(I) chain, Procollagen Type I, CT, Alpha-1 type I collagen
About This Item
Recommended Products
biological source
mouse
Quality Level
antibody form
purified immunoglobulin
antibody product type
primary antibodies
clone
PCIDG10, monoclonal
species reactivity
mouse, human, rat, guinea pig
species reactivity (predicted by homology)
bovine (based on 100% sequence homology)
technique(s)
ELISA: suitable
flow cytometry: suitable
immunocytochemistry: suitable
immunohistochemistry: suitable (paraffin)
isotype
IgG1κ
NCBI accession no.
UniProt accession no.
shipped in
dry ice
target post-translational modification
unmodified
Gene Information
human ... COL1A1(1277)
General description
Specificity
Immunogen
Application
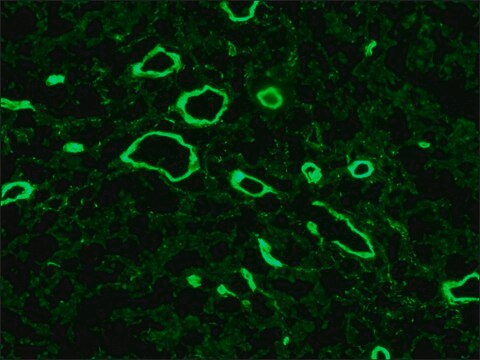
Immunocytochemistry Analysis: A representative lot detected type I procollagen immunoreactivity in human semitendinosus and gracilis tendon fibroblasts from patients undergoing reconstruction surgery after anterior cruciate ligament (ACL) rupture by fluorescent immunocytochemistry (Bayer, M.L., et al. (2012). Mech Ageing Dev. 133(5):246-254).
Flow Cytometry Analysis: A representative lot detected PICP+/CD45+ fibrocytes in lung cells from bleomycin-treated mice (Yeager, M.E., et al. (2012). Eur Respir J. 39(1):104-111).
Flow Cytometry Analysis: A representative lot detected higher numbers and percentages of circulating PICP+/CD45+ fibrocytes in peripheral blood samples from children/yound adults with pulmonary hypertension (PH) than in samples from healthy individuals (Reese, C., et al. (2014). Front Pharmacol. 5:141).
Immunohistochemistry Analysis: A representative lot detected cytoplasmic type I procollagen immunoreactivity in stromal cells from the lysed functionalis of frozen human menstrual endometria sections (Gaide Chevronnay, H.P., et al. (2009). Endocrinology. 150(11):5094-5105).
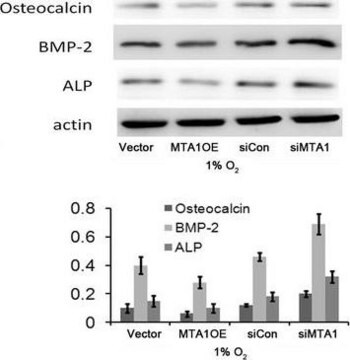
ELISA Analysis: A representative lot detected different age-dependency of procollagen type I C-terminal propeptide (PICP) immunoreactivity in the cruciate ligaments of osteoarthritis-/OA-prone Dunkin-Hartley (DH) guinea pigs and in age-matched Bristol strain 2 (BS2) control guinea pigs (Quasnichka, H.L., et al. (2005). Arthritis Rheum. 52(10):3100-3109).
Cell Structure
Adhesion (CAMs)
Quality
Immunohistochemistry Analysis: A 1:50 dilution of this antibody detected Procollagen Type I in human bone tissue.
Target description
Physical form
Storage and Stability
Handling Recommendations: Upon receipt and prior to removing the cap, centrifuge the vial and gently mix the solution. Aliquot into microcentrifuge tubes and store at -20°C. Avoid repeated freeze/thaw cycles, which may damage IgG and affect product performance.
Other Notes
Disclaimer
Not finding the right product?
Try our Product Selector Tool.
Storage Class Code
12 - Non Combustible Liquids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service