About This Item
Recommended Products
Quality Level
Assay
95%
form
solid
reaction suitability
reaction type: click chemistry
mp
45-50 °C
SMILES string
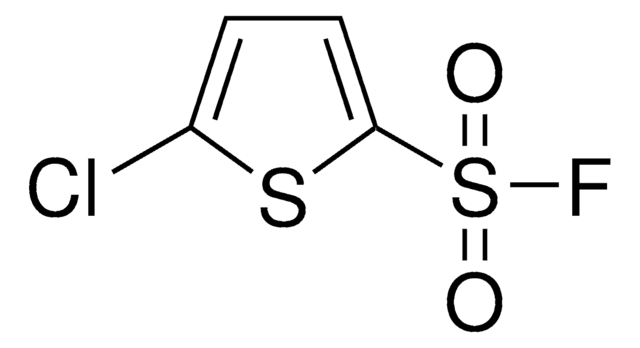
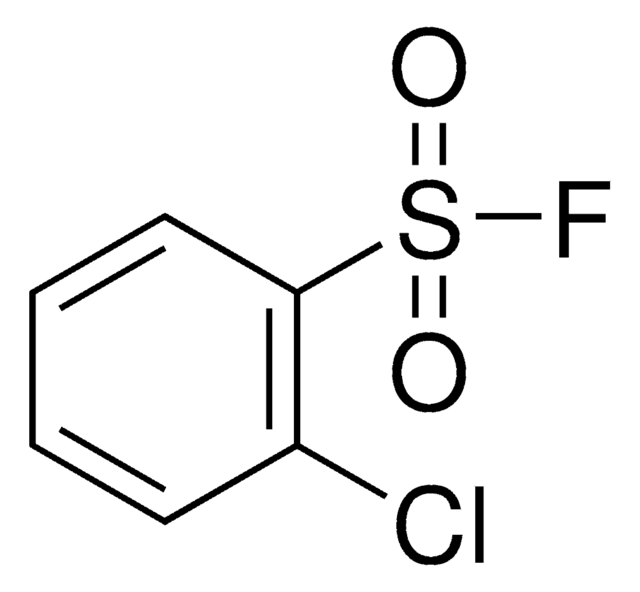
ClC1=CC=C(S(=O)(F)=O)C=C1
InChI
1S/C6H4ClFO2S/c7-5-1-3-6(4-2-5)11(8,9)10/h1-4H
InChI key
PCTLRVPDZBVCMP-UHFFFAOYSA-N
Application
This new click chemistry approach through sulfates is a complimentary approach to using amides and phosphate groups as linkers.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
This article details the latest progress in synthesizing alkylsulfonyl fluorides through different approaches that utilize photoredox catalysis, electrocatalysis, transition-metal catalysis, and organocatalysis. The alkylsulfonyl fluorides thus prepared could be utilized further in sulfur(VI) fluoride exchange (SuFEx) click reactions.
This article details the latest progress in synthesizing alkylsulfonyl fluorides through different approaches that utilize photoredox catalysis, electrocatalysis, transition-metal catalysis, and organocatalysis. The alkylsulfonyl fluorides thus prepared could be utilized further in sulfur(VI) fluoride exchange (SuFEx) click reactions.
This article details the latest progress in synthesizing alkylsulfonyl fluorides through different approaches that utilize photoredox catalysis, electrocatalysis, transition-metal catalysis, and organocatalysis. The alkylsulfonyl fluorides thus prepared could be utilized further in sulfur(VI) fluoride exchange (SuFEx) click reactions.
This article details the latest progress in synthesizing alkylsulfonyl fluorides through different approaches that utilize photoredox catalysis, electrocatalysis, transition-metal catalysis, and organocatalysis. The alkylsulfonyl fluorides thus prepared could be utilized further in sulfur(VI) fluoride exchange (SuFEx) click reactions.
Related Content
The Sharpless Lab pursues useful new reactivity and general methods for selectively controlling chemical reactions.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service